Exiguobacterium sp. MY02 strain pyrimidine nucleoside phosphorylase gene and its preparation method
A pyrimidine nucleoside phosphorylase gene and technology of pyrimidine nucleoside phosphorylase, applied in the field of pyrimidine nucleoside phosphorylase gene, can solve the problems of unsuitable industrial production and low transferase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: Cloning and sequencing of pyrimidine nucleoside phosphorylase gene
[0175] The genomic DNA library of the Exiguobacterium sp.MY02 strain was screened by PCR technology, and the unknown pyrimidine nucleoside phosphorylase gene was cloned. The specific operation was: in Luria-Bertani (LB) medium containing 1% NaCl weight percentage concentration The Exiguobacterium sp.MY02 was cultured to the end logarithmic phase, and the genomic DNA was extracted. The genomic DNA was digested with Sau3A I, electrophoresed on agarose gel, and a 5-8 kb fragment was recovered, which was ligated with the pUC18 plasmid fragment completely digested with BamH I and dephosphorylated. Then, the ligation product was transferred into Escherichia coli DH5α competent cells according to the standard calcium chloride method, and cultured on LB solid medium (containing ampicillin, X-gal, IPTG). Pick out white spot recombinant colonies, culture them in LB liquid medium (containing ampicilli...
Embodiment 2
[0176] Embodiment 2: Construction of Escherichia coli expression vector
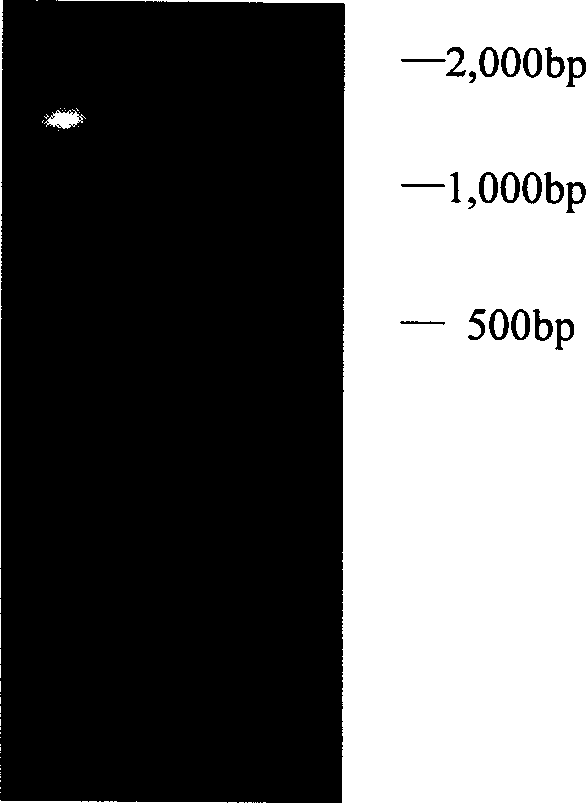
[0177] The upstream primer (5'GCACCATGGTAA TGCGTATGGTAGATTTG3') and the downstream primer (5'CCGTCTAGAAAATTGAATGACAGCGTGGAC') were designed according to the obtained pyrimidine nucleoside phosphorylase gene sequence, and the complete sequence of the pyrimidine nucleoside phosphorylase gene was obtained by PCR amplification. The PCR conditions were as follows: pre-denaturation at 94°C for 2 minutes, followed by 30 cycles of 94°C for 30s, 60°C for 30s, and 72°C for 90s, and finally extension at 72°C for 10 minutes. Agarose gel electrophoresis showed a specific band at 1.3 kb, which was excised from the agarose gel, digested with Nco I and Xba I, and a 1.3 kb DNA fragment was recovered after agarose gel electrophoresis.
[0178] The Escherichia coli expression vector pBAD gIII / A was also digested with Nco I and Xba I, separated by agarose electrophoresis, and a 4.1kb DNA fragment was recovered, which was li...
Embodiment 3
[0179] Example 3: Construction of an Escherichia coli engineering strain capable of highly expressing pyrimidine nucleoside phosphorylase
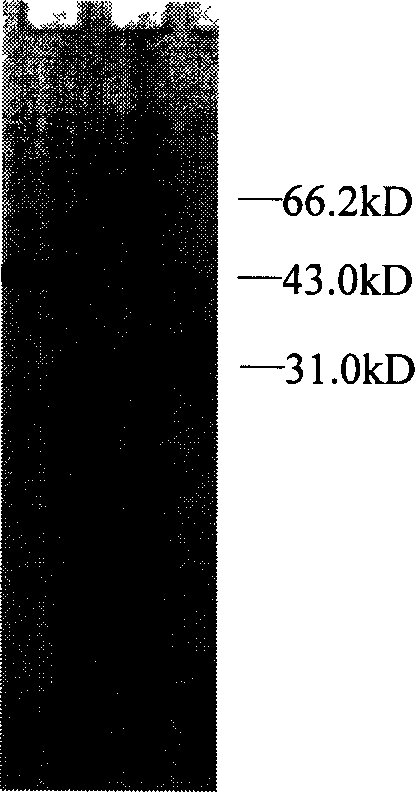
[0180] The expression vector pPNP-A was transformed into Escherichia coli Top10F' according to the standard calcium chloride method, and the transformant with ampicillin resistance was screened. The plasmid was extracted by a standard alkaline lysis method, and two fragments of 1.3kb and 4.1kb were obtained by digestion with NcoI and XbaI, which were respectively the same size as the pyrimidine nucleoside phosphorylase gene and the expression vector pBAD gIII / A, proving that the highly efficient Escherichia coli recombinant strain pLyase-A / Top10F' expressing pyrimidine nucleoside phosphorylase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com