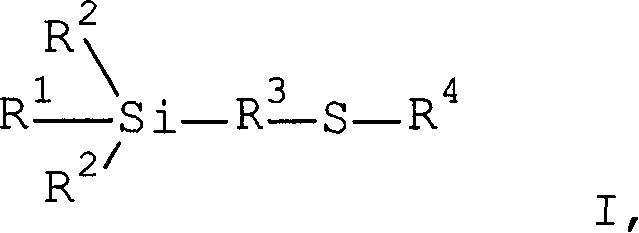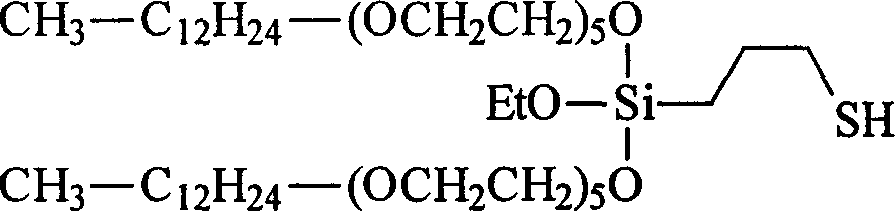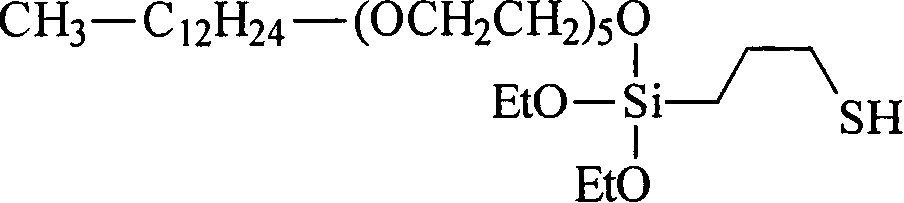Mercapto silane
A technology of mercaptosilane and group, applied in the field of mercaptosilane, can solve the problems of impossible to omit mercaptosilane, short mixing time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0537] Weigh 59.63g (0.25mol) of 3-mercaptopropyltriethoxysilane (VP Si 263, available from Degussa AG), 212.92g (0.50mol) of ethoxylated alcohol R 1 H, where R 5 Is CH 2 -CH 2 , R 6 It is an unsubstituted monovalent alkyl group containing 13 carbon atoms with an average m of 5 (Lutensol TO5, available from BASF AG), and 30μl of titanium tetrabutoxide, and placed in a 500ml four-necked flask at room temperature under a nitrogen atmosphere , It is equipped with distillation bridge, KPG stirrer and thermometer. The mixture was heated to 140°C. The ethanol formed is continuously distilled off. After 35 minutes, the reduced pressure was adjusted to 640 mbar and reduced to 50 mbar over the course of 3 hours. The reaction was terminated after 3 hours and 35 minutes. Obtained 245.37 g (98.6%) of turbid and light yellow product. by 1 The H-NMR spectrum gave an average transesterification degree of 2.0. by 13 C-NMR can determine the distribution of long-chain branched alkyl polyethers on S...
Embodiment 2
[0538] Example 2 (comparative example)
[0539] Weigh 2,925.3g of 3-mercaptopropyltriethoxysilane, 4,753.4g of an alcohol mixture containing 72% dodecanol and 28% tetradecanol, and 30μl of titanium tetrabutoxide, and place them at room temperature under a nitrogen atmosphere. Put it into a 4l four-necked flask equipped with distillation bridge, KPG stirrer and thermometer. The mixture was heated to 110°C. The ethanol formed is continuously distilled off. After 2 hours, the reduced pressure was continuously reduced to 50 mbar during the course of 3 hours. When 1,140 ml of ethanol was removed from the reaction mixture, the reaction was terminated. 6.47 kg (98.6%) of light yellow liquid was obtained. by 1 The H-NMR spectrum gave an average transesterification degree of 2.0.
Embodiment 3
[0540] Example 3 (comparative example)
[0541] Weigh 150.02g (0.63mol) of 3-mercaptopropyltriethoxysilane, 151.2g (1.26mol) of diethylene glycol monomethyl ether and 75μl of titanium tetrabutoxide, and put them in a nitrogen atmosphere at room temperature In a 500ml three-necked flask, it is equipped with an enhanced condenser, a stirrer and a thermometer. The mixture was heated to 80°C. The ethanol formed was removed under a reduced pressure of 3 mbar.
[0542] The reaction was terminated after 8 hours. Obtained 237.84 g (97.6%) of a clear and light yellow product. by 1 The H-NMR spectrum gave an average transesterification degree of 2.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com