Method for determining content of high molecule weight substance in injection
A technology of high molecular weight and substance content, applied in the direction of measuring device, material separation, analysis material, etc., to achieve the effect of less interference, saving first aid time, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Determination of high molecular weight substance content by high performance liquid chromatography
[0026] 1. Test materials and instruments
[0027] name
[0028] 2. Instrument
[0029] Visible ultraviolet spectrophotometer (Hitachi U-3210, Japan)
[0030] High performance liquid chromatography Waters 600 system
[0031] High performance liquid chromatography Agilent Hp1100
[0032] 3. Reagents
[0033] Chemically pure trifluoroacetic acid (School of Chemical Engineering and Materials, Polytechnic University)
[0034] Chromatographically pure acetonitrile (Dima Company)
[0035] 2. Chromatographic conditions
[0036] Chromatographic column: gel chromatography column (TSK GEL 2000 SWxl 7.8mm×300mm);
[0037] Mobile phase: trifluoroacetic acid-acetonitrile-water, the volume ratio is 0.025:30:70;
[0038] Ultraviolet detector, the detection wavelength is 214±1nm;
[0039] The flow rate was 0.7ml / min.
[0040] 3. Determination
[0041] T...
Embodiment 2
[0044] Embodiment 2: repeatability test
[0045] Take an appropriate amount of human insulin standard, add mobile phase to dilute to 3 different concentrations, measure 3 times respectively, the results are shown in Table 2.
[0046] concentration
[0047] The test results show that the method of the present invention has very good repeatability.
Embodiment 3
[0048] Embodiment 3: Determination of high molecular weight substance content in Shuxuetong injection by high performance liquid chromatography
[0049] Preparation of test product solution: get test product Shuxuetong injection (batch numbers 0212031 and 0212051, specification 2ml / support, produced by Mudanjiang Youbo Pharmaceutical Co., Ltd.), add mobile phase to dilute 5 times, shake well, and use as test product solution .
[0050] Preparation of reference substance solution: take an appropriate amount of human insulin (molecular weight 5800), and use mobile phase to make a solution containing 0.1 mg in every 1 ml, as the reference substance solution.
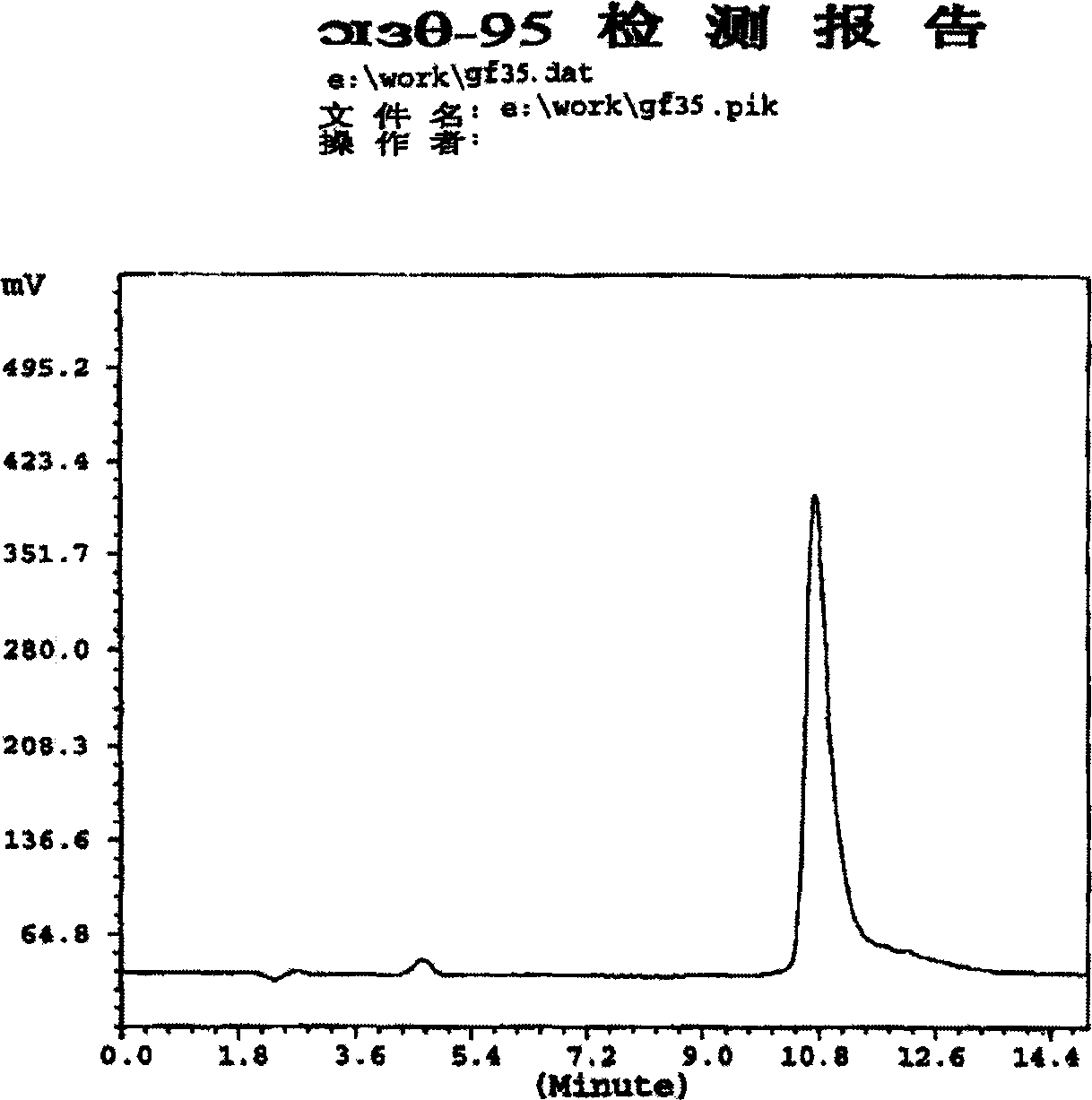
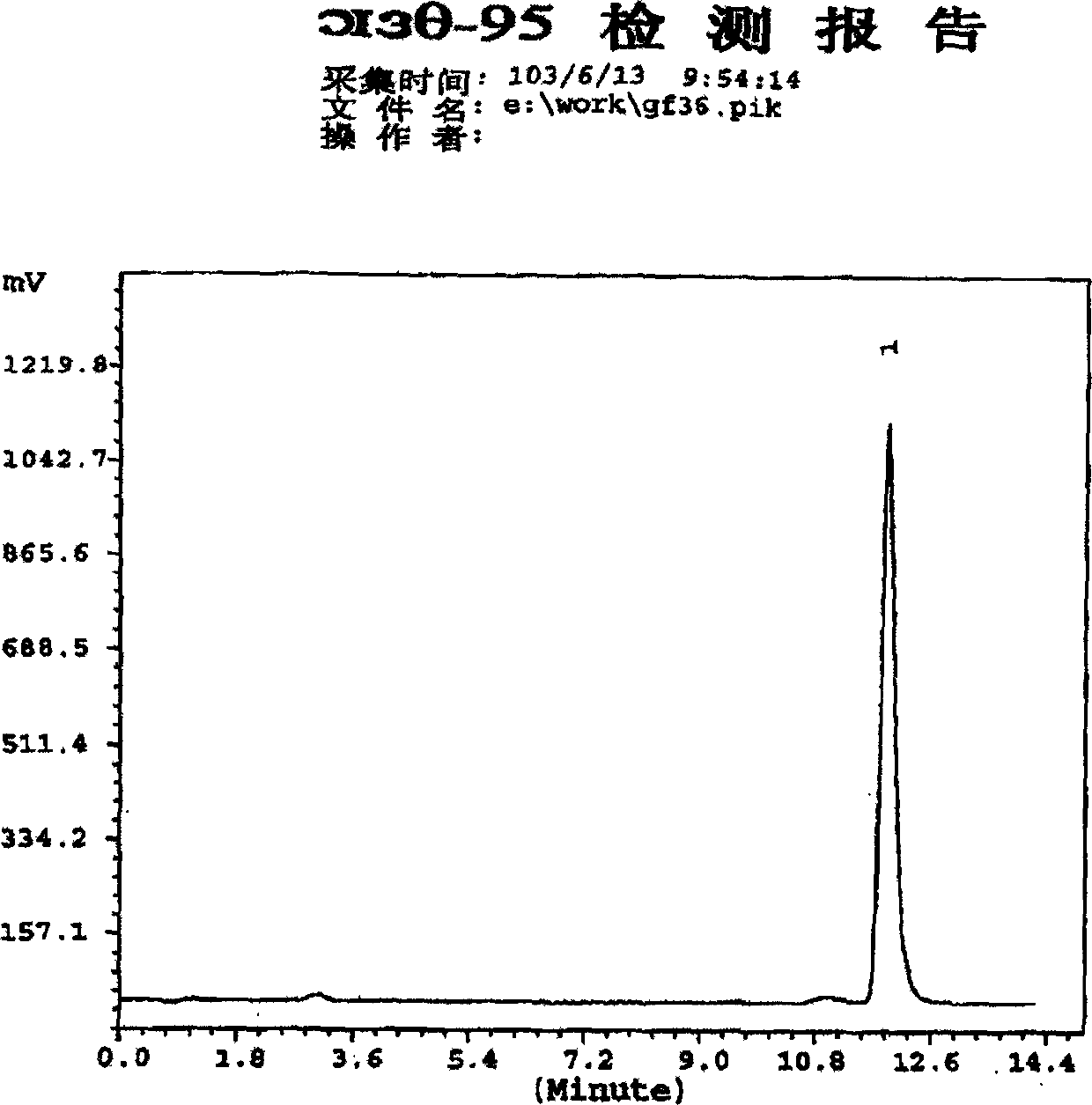
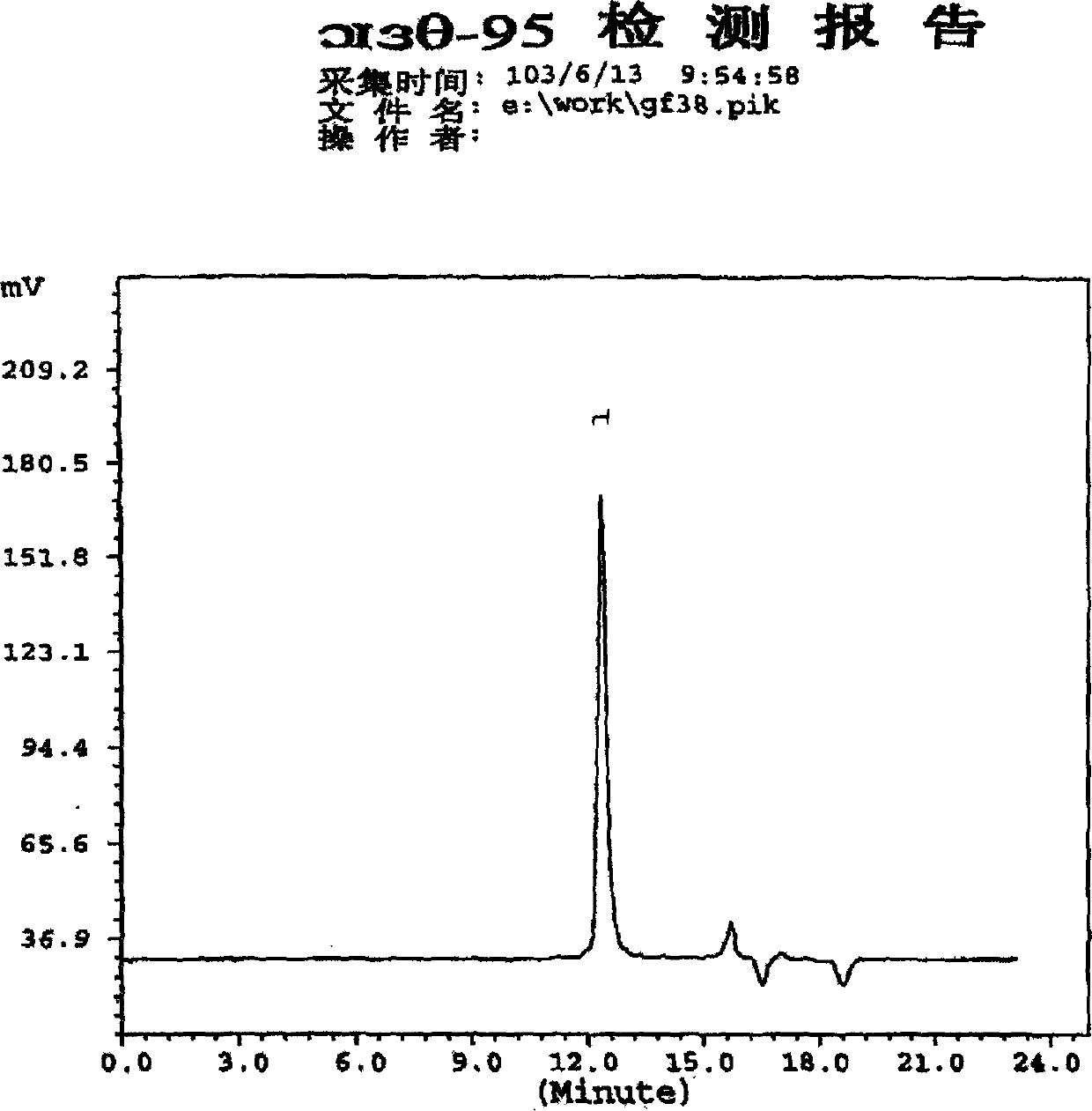
[0051] Determination: Take 20ul each of the reference substance solution and the test solution and inject them into the high-performance liquid chromatograph respectively, record the chromatogram, regard the peak prior to the insulin peak retention time as the high molecular weight substance peak, and calculate according to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com