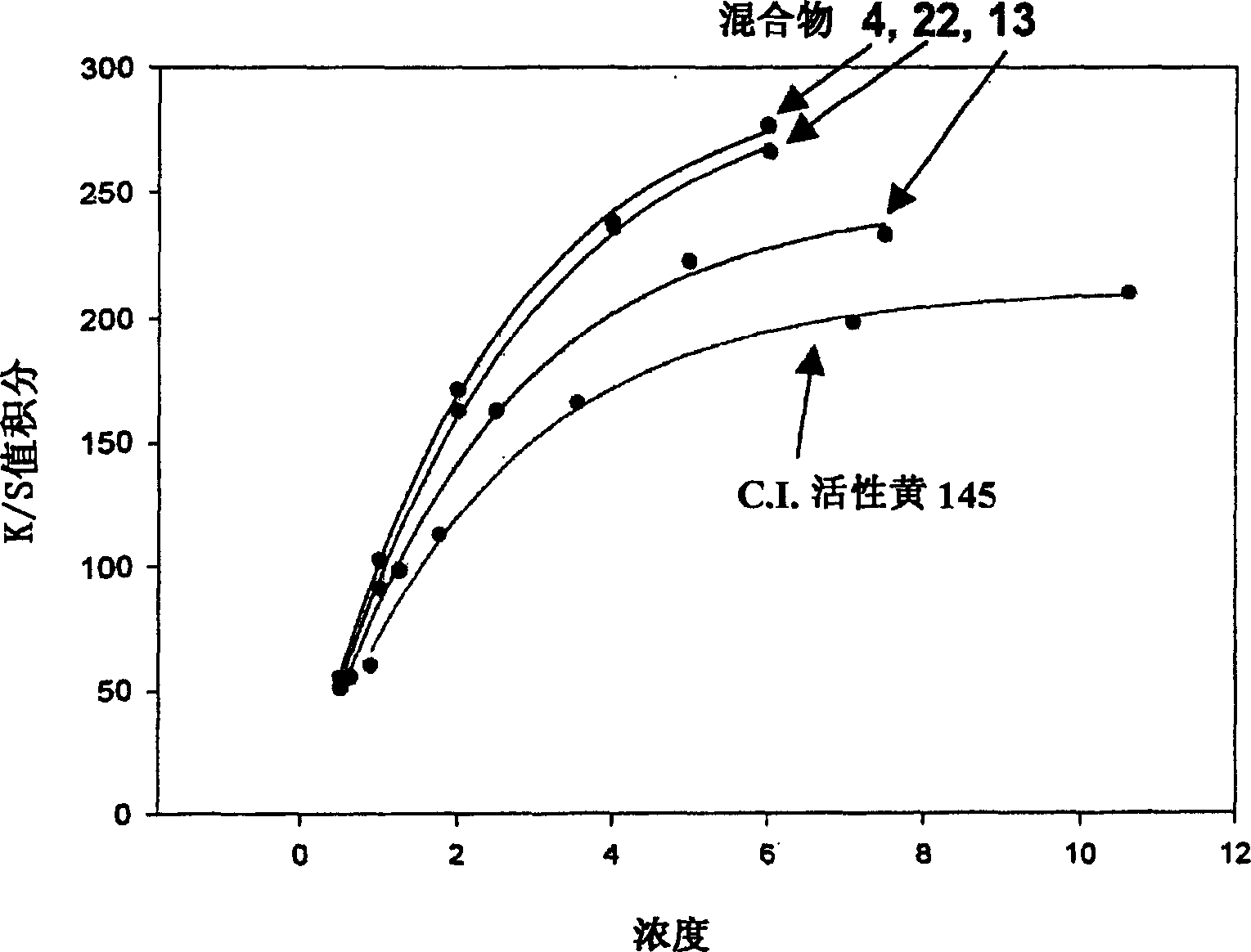
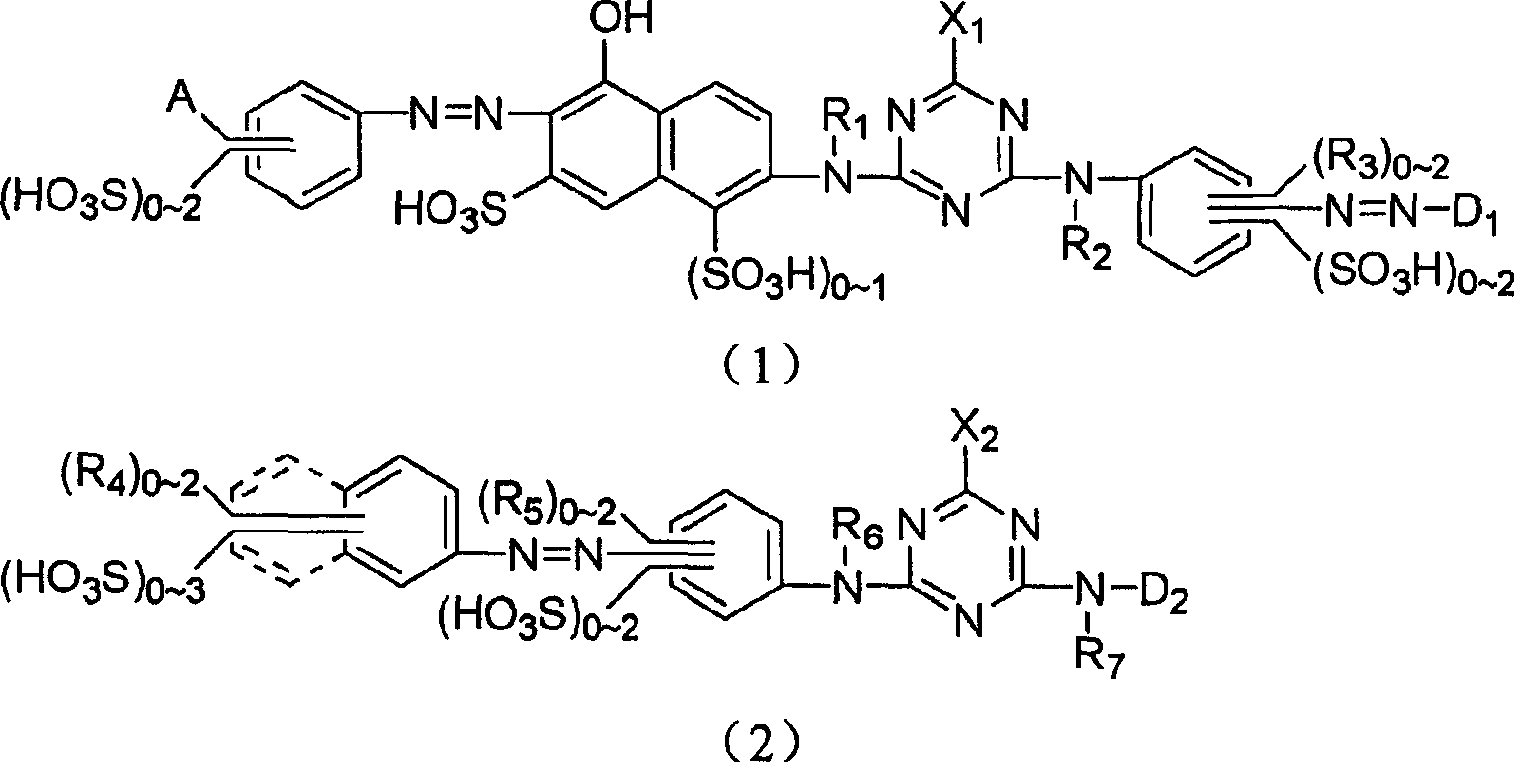
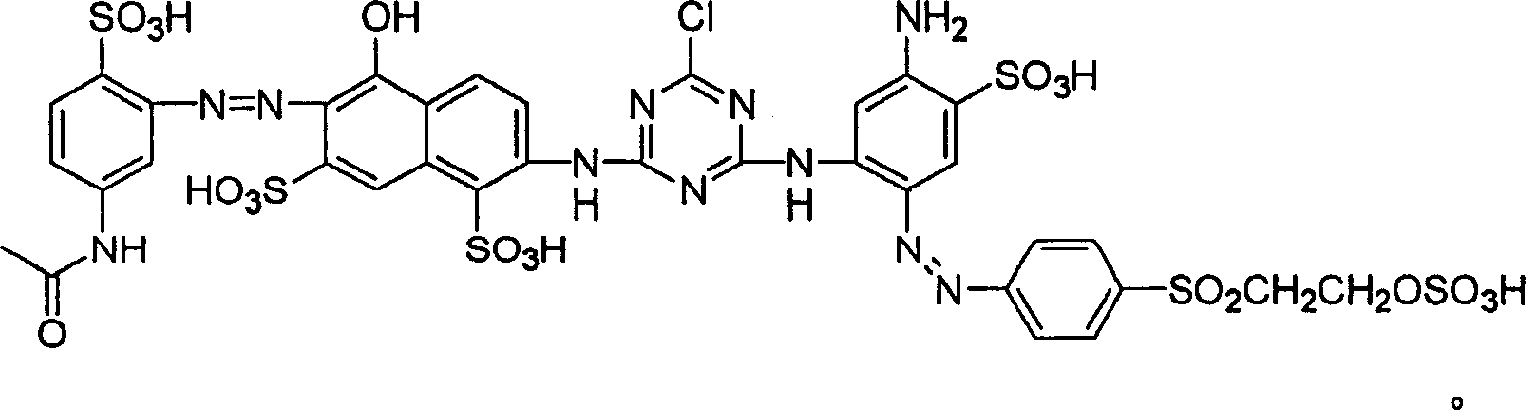
Novel reactive yellow dye compounds and mixture thereof
A technology of reactive dyes and mixtures, applied in the field of color-blocking dyeing, can solve the problems of weak compatibility, low lifting force, unsatisfactory dyeing with high concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0069] 239.3 parts of 2-amino-naphthol-7-sulfonic acid were dispersed in 2000 parts of water, and the resulting dispersion was neutralized to pH 7 using 25% aqueous sodium hydroxide. 184.4 parts of cyanuric chloride were dispersed in 300 parts of water at 0°C, and then the above-mentioned 2-amino-naphthol-7-sulfonic acid solution was added dropwise thereto for 1 hour. The resulting solution was neutralized to pH 3.0 using a 20% aqueous sodium carbonate solution, thereby producing a first condensation product.
[0070] 23.12 parts of (4-N-acetyl)-2,4-phenylenediaminesulfonic acid were dispersed in 50 parts of water, and 156.4 parts of 35% hydrochloric acid were added thereto. Then, 230 parts of 30% sodium nitrite solution was added dropwise to the resulting solution for 30 minutes while keeping the temperature below 5° C., and the mixture was maintained at this temperature for 2 hours to synthesize (4-N-acetyl) - The diazonium salt of 2,4-phenylenediaminesulfonic acid.
[007...
preparation Embodiment 2
[0077] 319.3 parts of 2-amino-naphthol-1,7-disulfonic acid were dispersed in 1500 parts of water, and the resulting dispersion was neutralized to pH 7 using 25% aqueous sodium hydroxide. 184.4 parts of cyanuric chloride were dispersed in 300 parts of water at 0°C, and then a 2-amino-naphthol-7-sulfonic acid solution was added dropwise thereto for 1 hour. The resulting solution was neutralized to pH 3.0 using a 20% aqueous sodium carbonate solution, thereby producing a first condensation product.
[0078]23.12 parts of (4-N-acetyl)-2,4-phenylenediaminesulfonic acid were dispersed in 50 parts of water, and 156.4 parts of 35% hydrochloric acid were added thereto. Then, 230 parts of 30% sodium nitrite solution was added dropwise to the resulting solution in 30 minutes while keeping the temperature lower than 5° C., and the mixture was maintained at this temperature for 2 hours, thereby synthetically obtaining (4-N-acetyl )-2,4-diazonium salt of phenylenediaminesulfonic acid.
[...
preparation Embodiment 3-86
[0085] Based on the methods in Preparation Examples 1 and 2, the compounds listed in Table 1 could be synthesized. Based on the chemical structures of these products, the specific preparation methods of these compounds can be fully deduced from Preparation Examples 1 and 2, and thus details thereof are omitted here.
[0086] Formula 1 compound
[0087]
[0088] Table 1
[0089]
[0090]
[0091]
[0092]
[0093]
[0094]
[0095]
[0096]
[0097]
[0098]
[0099]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com