Method for the detection of a pathogenic form of a prion protein
A prion protein, prion technology, used in biochemical equipment and methods, disease diagnosis, biological testing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
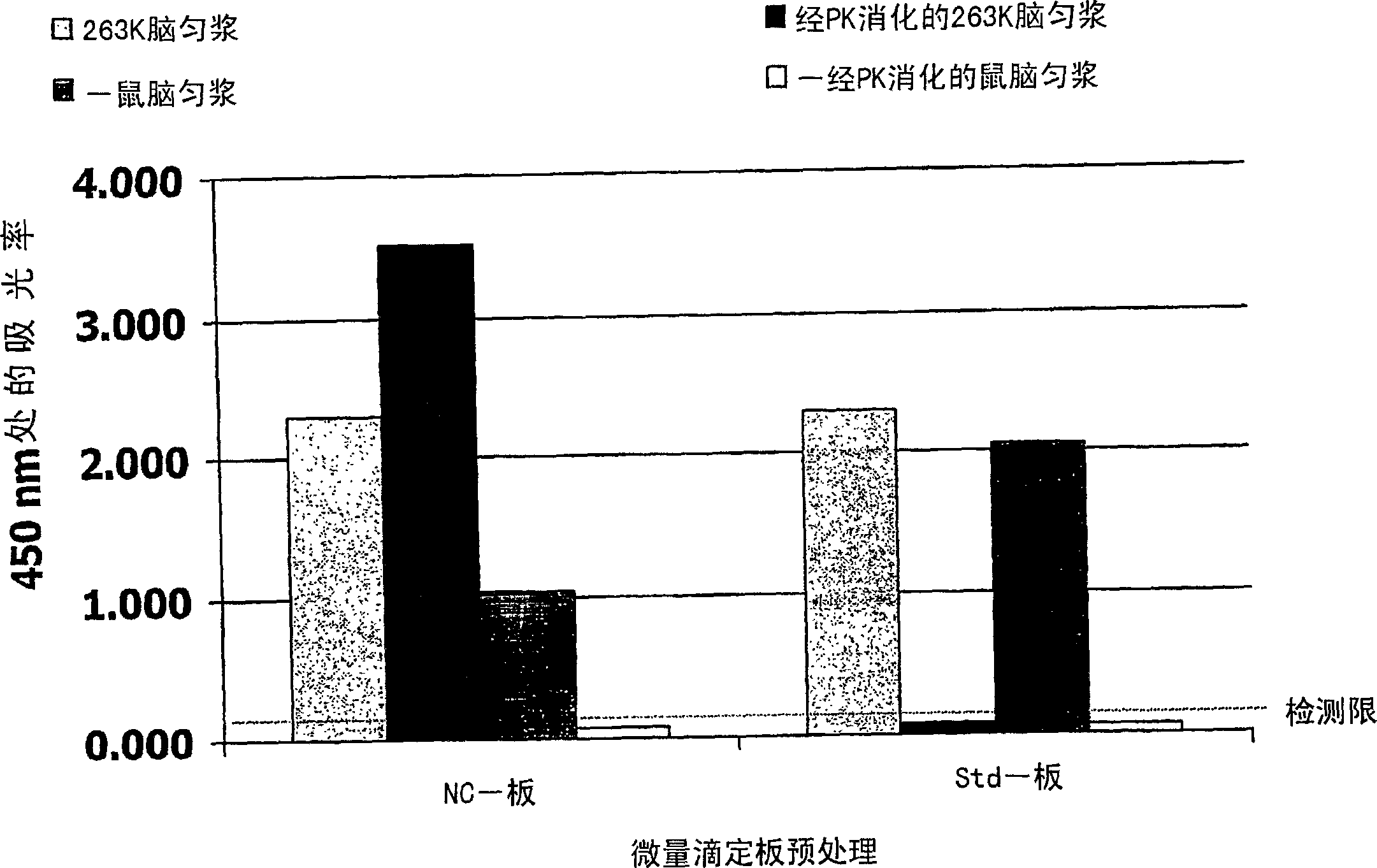
[0054] In order to demonstrate the effect of a clear nitrocellulose coating on the prion-binding properties of microtiter plates, applicants performed experiments with nitrocellulose-coated microtiter plates (NC-plates) or standard microtiter plates (Std-plates). Direct ELISA. Homogenates (0.1%) of scrapie-infected hamster brains (263K strain) or normal mouse brains were treated with or without PK; and performed on NC-plates (0.1% nitrocellulose-coated) or Std-plates Incubation. Bound prion protein was detected with horseradish peroxidase (HRP)-labeled anti-prion antibody 1E4, followed by staining with 3,3',5,5'-tetramethylbenzidine (TMB) substrate.
[0055] as in figure 1 Prion protein (PrP) present in the brain of scrapie-infected hamsters digested by PK Sc ) binds to the NC-plate, but not to the Std-plate. In addition, prion protein (PrP) present in undigested normal mouse brain C ) binds weaker to the NC-plate than to the Std-plate.
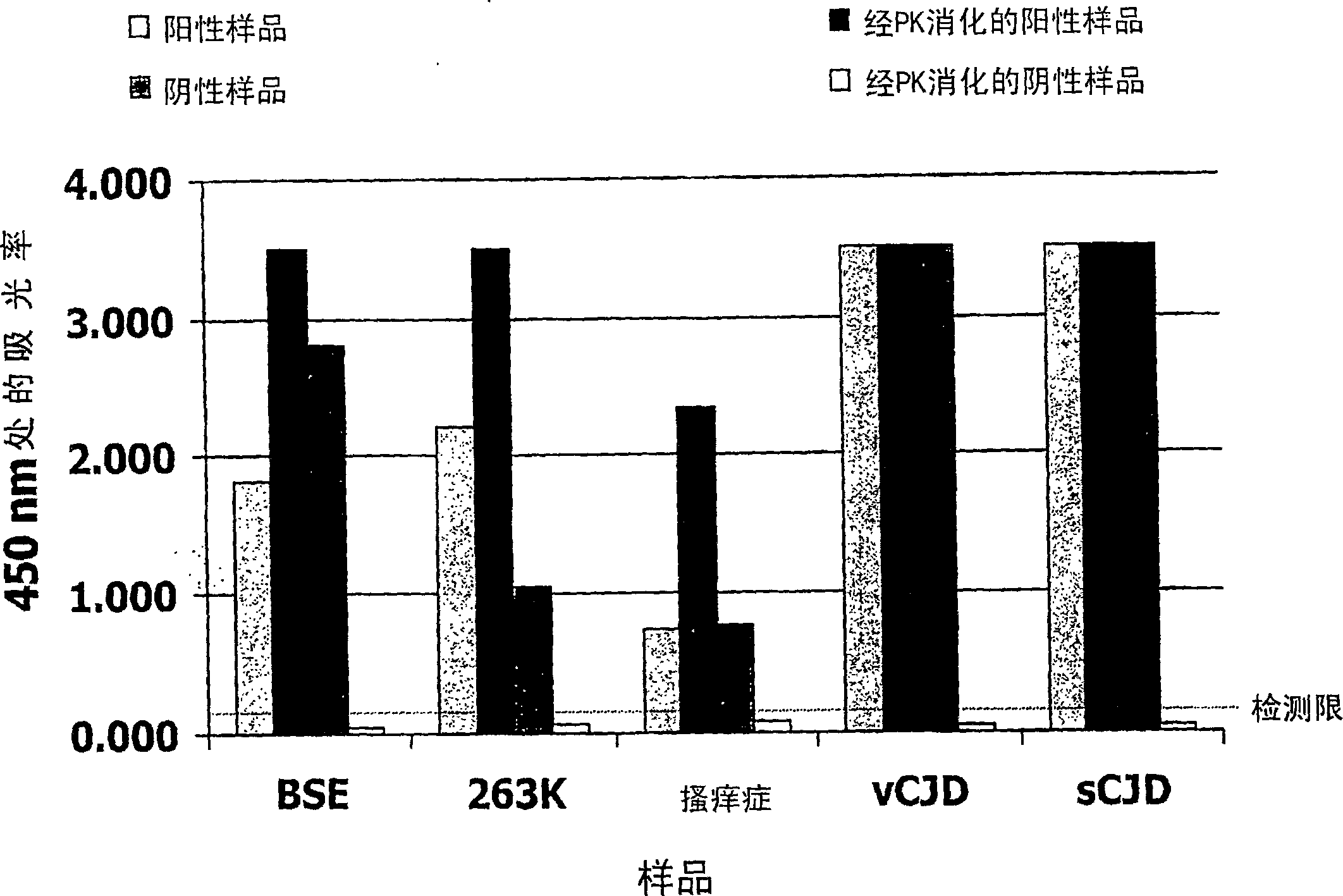
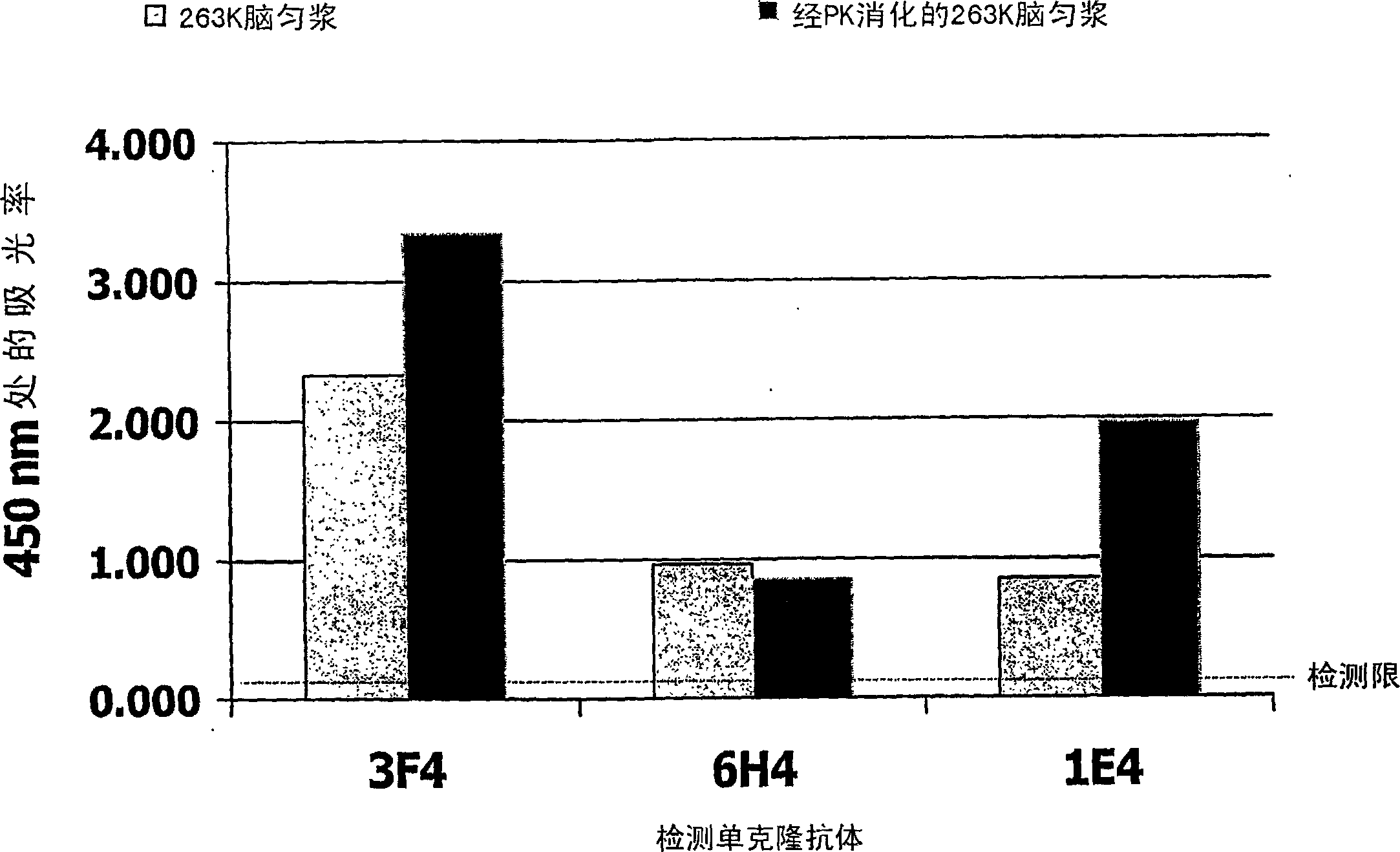
[0056] In order to demonstrate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com