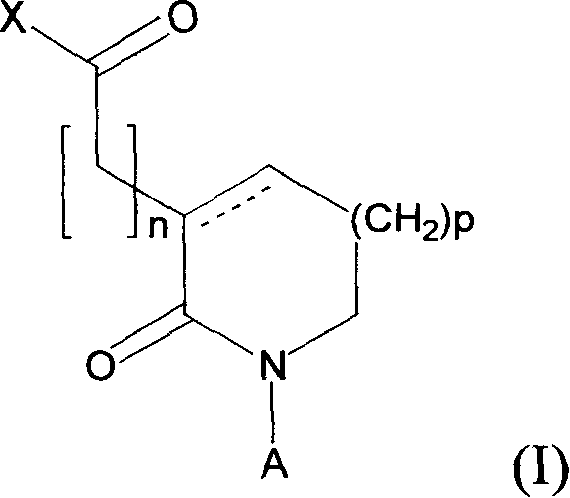
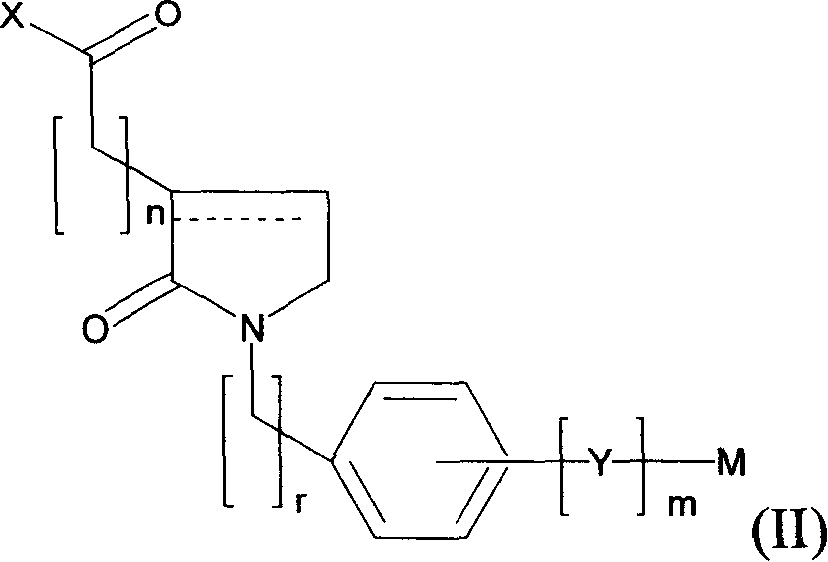
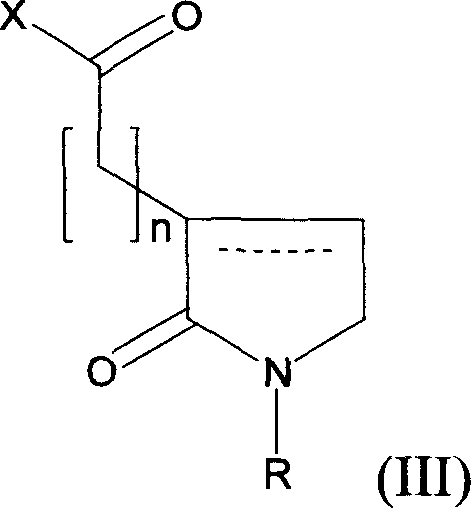
Use of novel 2-oxo-heterocyclic compounds and the pharmaceutical compositions comprising the same
A compound and composition technology, applied in the fields of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0228] Example 1. Preparation of 3-[1-(2,4-dimethoxybenzyl)-2-oxo-2,5-dihydro-1H-pyrrol-3-yl]-N-hydroxyl propionamide ( 1e)
[0229]
[0230] Step 1. Preparation of Allyl-(2,4-Dimethoxybenzyl)amine (1b)
[0231] Under stirring, 0.32 ml of allyl bromide (3.66 mM) and 0.7 ml of diisopropylethylamine (3.99 mM) were added to 500 mg of 2,4-dimethoxybenzyl dissolved in dichloromethane In the reaction solution of amine (3.33 mM), the solution was left alone at room temperature. After neutralizing the reaction mixture with 10% NaOH solution, the mixture was extracted with chloroform, washed with saturated NaCl solution, washed with MgSO 4 Dry, filter, and concentrate in vacuo. The formed compound was purified by silica gel column chromatography using a solvent mixture of methanol and chloroform (1:9) as eluent to obtain 276 mg of allyl-(2,4-dimethoxybenzyl)amine (1b) ( Yield: 40%).
[0232] 1 H-NMR (300MHz, CDCl 3 )δ7.12(d, J=8.1Hz, 1H), 6.44-6.39(m, 2H), 5.99-5.86(m, 1H), 5...
Embodiment 2
[0242] Example 2. Preparation of 3-(1-benzyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)-N-hydroxyl-propionamide (2e)
[0243] 3-(1-Benzyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)-N-hydroxy-propionamide (2e) was prepared analogously to that described in Example 1 above (See Table 1).
Embodiment 3
[0244] Example 3. Preparation of N-hydroxy-3-(2-oxo-1-phenethyl-2,5-dihydro-1H-pyrrol-3-yl)-propionamide (3e)
[0245] N-Hydroxy-3-(2-oxo-1-phenethyl-2,5-dihydro-1H-pyrrol-3-yl)-propionamide (3e ) (see Table 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com