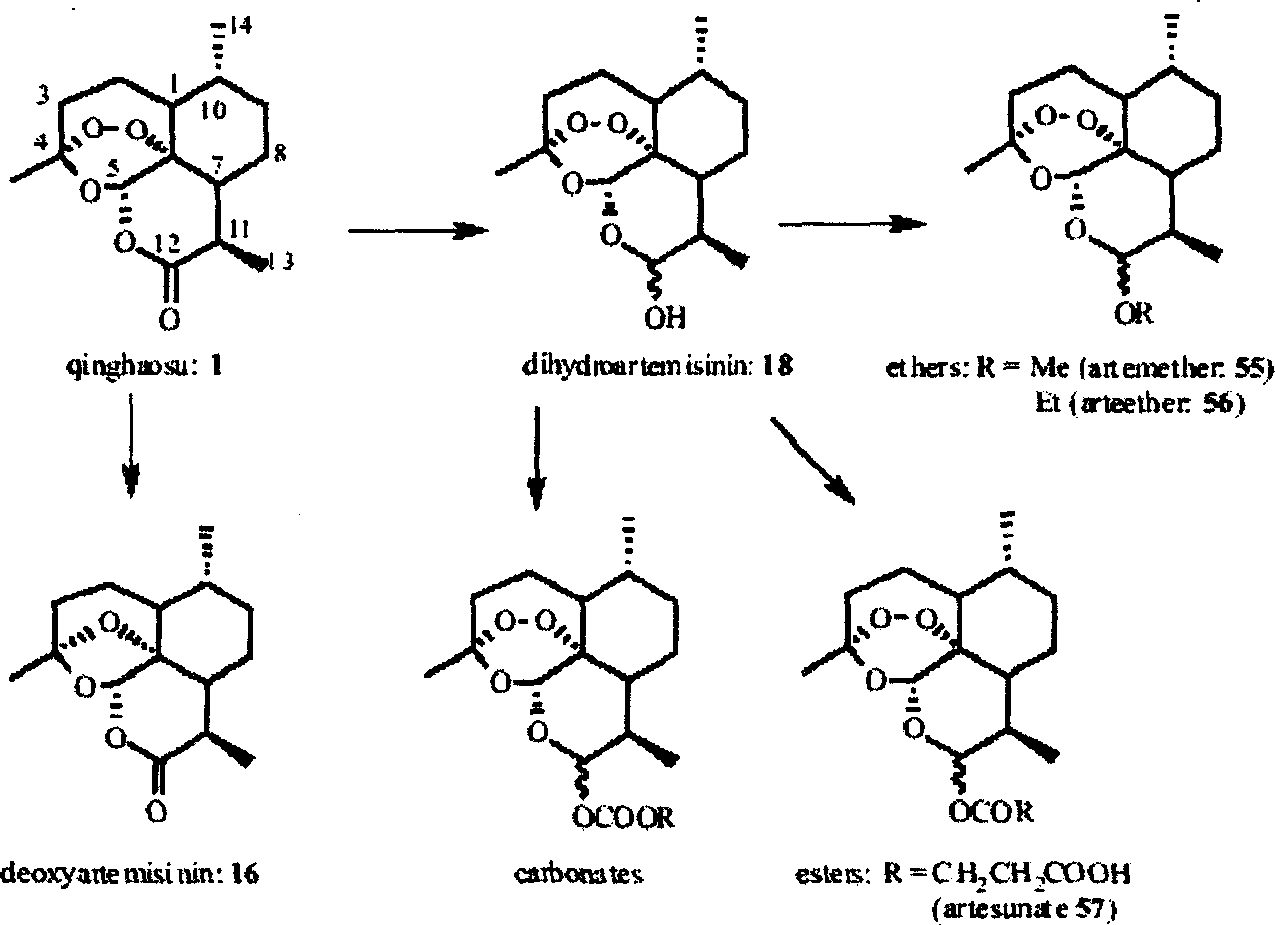
Abrotine, its derivative dihydro-abrotine, artemether, arteether and arte sunate in use of pharmacy
A technology of dihydroartemisinin and artesunate, which is applied in the pharmaceutical field to achieve strong pharmacological effects, good medicinal prospects, and the effect of preventing or treating sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental example 1. The dose-effect relationship of artemisinin, dihydroartemisinin, artemether and artesunate in inhibiting the release of cytokines induced by CpG-ODN.
[0023] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and different concentrations of artemisinin (5, 10, 20, 40, 80 μg / ml) or dihydroartemisinin, artemether, and artesunate were added. Add 10 μg / ml stimulatory CpG ODN (5′-TCC ATG ACG TTC CTG ACG TT-3′), culture in 37°C, CO2 incubator for 4 hours, take cell culture supernatant to test cytokine TNF-α, and then After 4 hours, the cell culture supernatant was taken to test the cytokine IL-6. The concentration of TNF-α and IL-6 in the cell culture supernatant was measured by double-antibody sandwich ELISA method, and the effect of artemisinin or its deri...
experiment example 2
[0027] Experimental example 2. The dose-effect relationship of artemisinin, dihydroartemisinin, artemether and artesunate in inhibiting the release of cytokines induced by heat-killed Escherichia coli.
[0028] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and different concentrations of artemisinin (5, 10, 20, 40, 80 μg / ml) or 40 μg / ml of dihydroartemisinin, artemether or artesunate were added, After 2 hours add 1 x 10 6 / ml heat-inactivated Escherichia coli, cultured in a 37°C, CO2 incubator for 4 hours, then took the cell culture supernatant to test the cytokine TNF-α, and after another 4 hours, took the cell culture supernatant to test the cytokine IL-6. The concentration of TNF-α and IL-6 in the cell culture supernatant was measured by double-antibody sandwich ELISA method,...
experiment example 3
[0033]Experimental example 3. Time-effect relationship of artemisinin inhibiting cytokine release induced by CpG ODN.
[0034] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C, CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and the time of adding CpG ODN was regarded as the 0 time point, and 20 μg / ml artemisinin was added at -4, -2, -1, 0, 1, and 2 hours respectively, and placed at 37°C, After 4 hours of culture in a CO2 incubator, the cell culture supernatant was taken to measure TNF-α, and after another 4 hours, the cell culture supernatant was taken to test the cytokine IL-6. To clarify the ability of artemisinin to inhibit the release of cytokines from RAW264.7 induced by CpG ODN.
[0035] point in time
Cytokines
TNF-α
(pg / ml)
IL-6
(pg / ml)
-4h
-2h
-1h
0h
1h
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com