Patents
Literature
62 results about "Dihydroarteannuin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
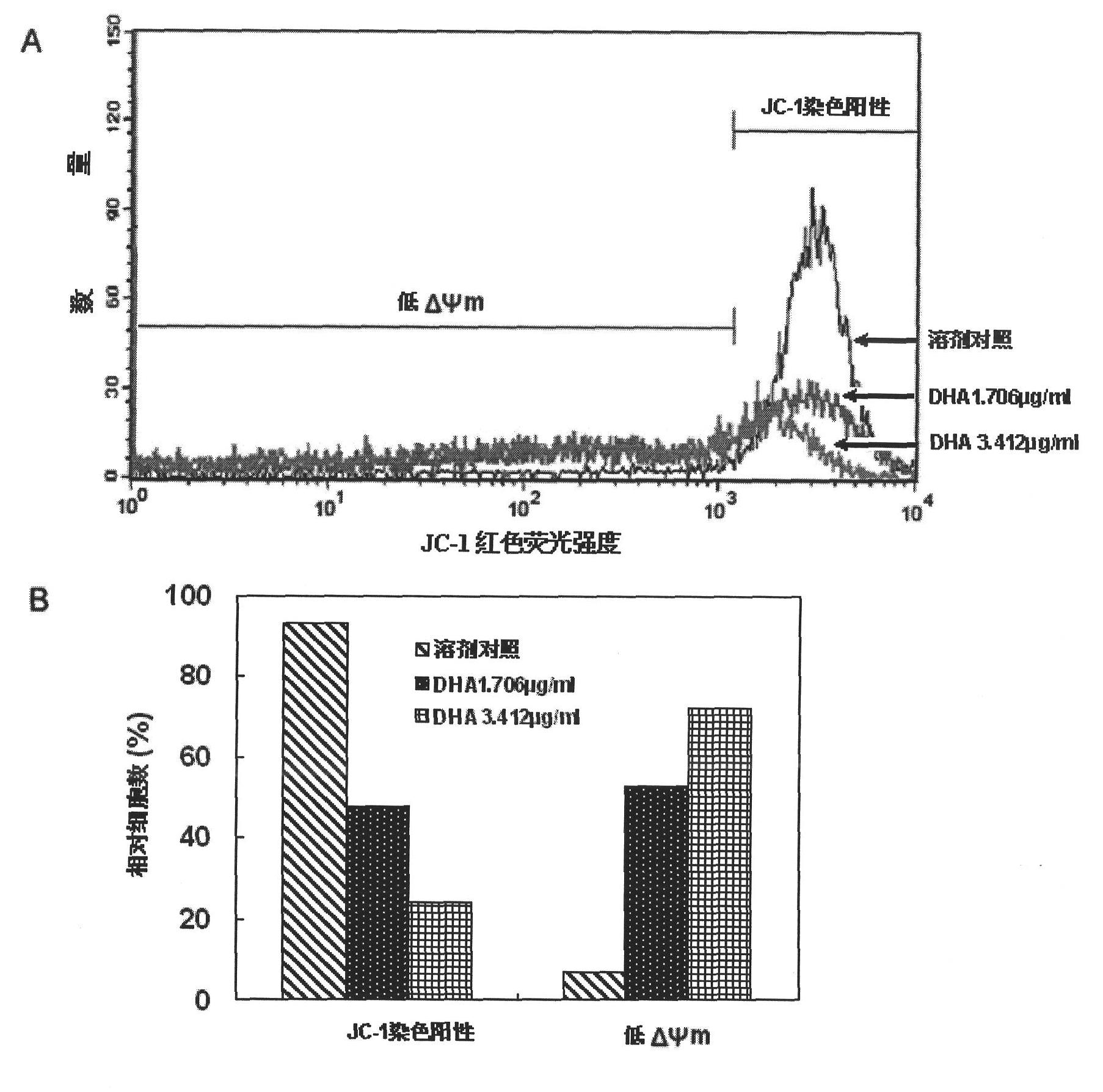
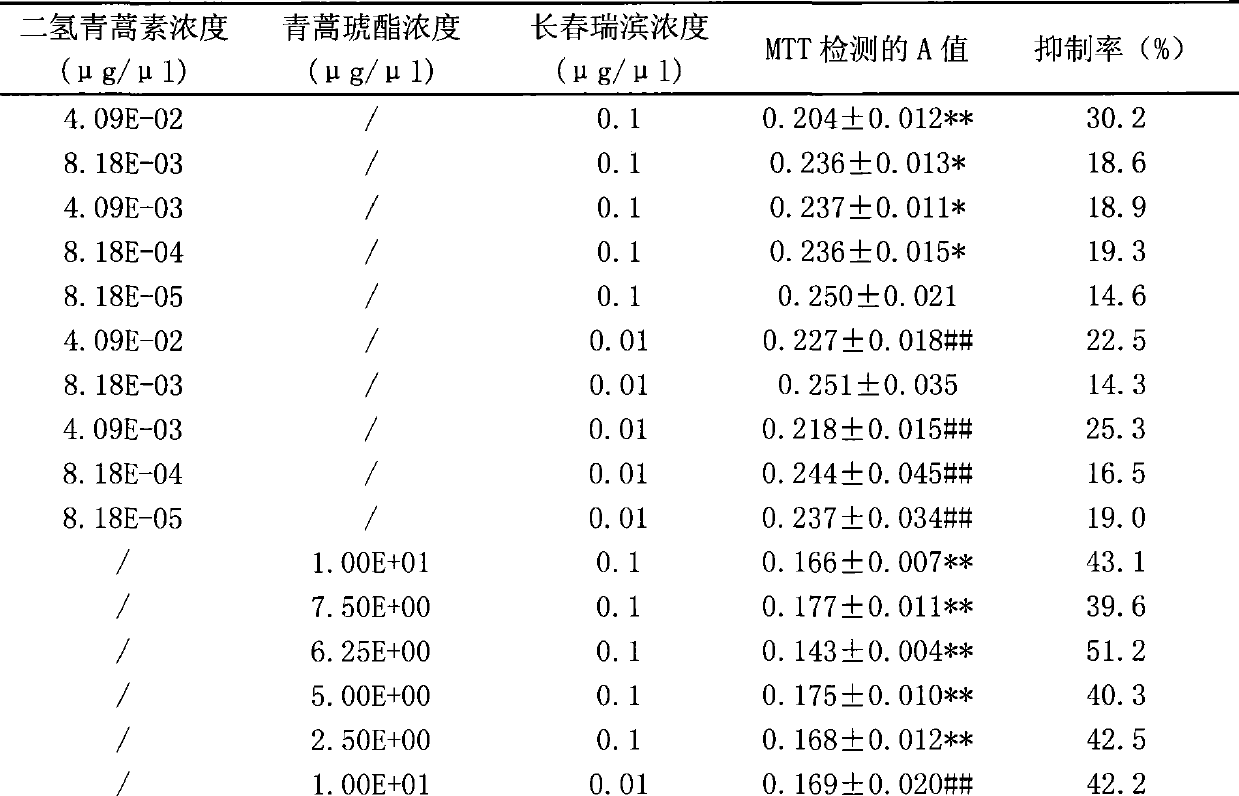
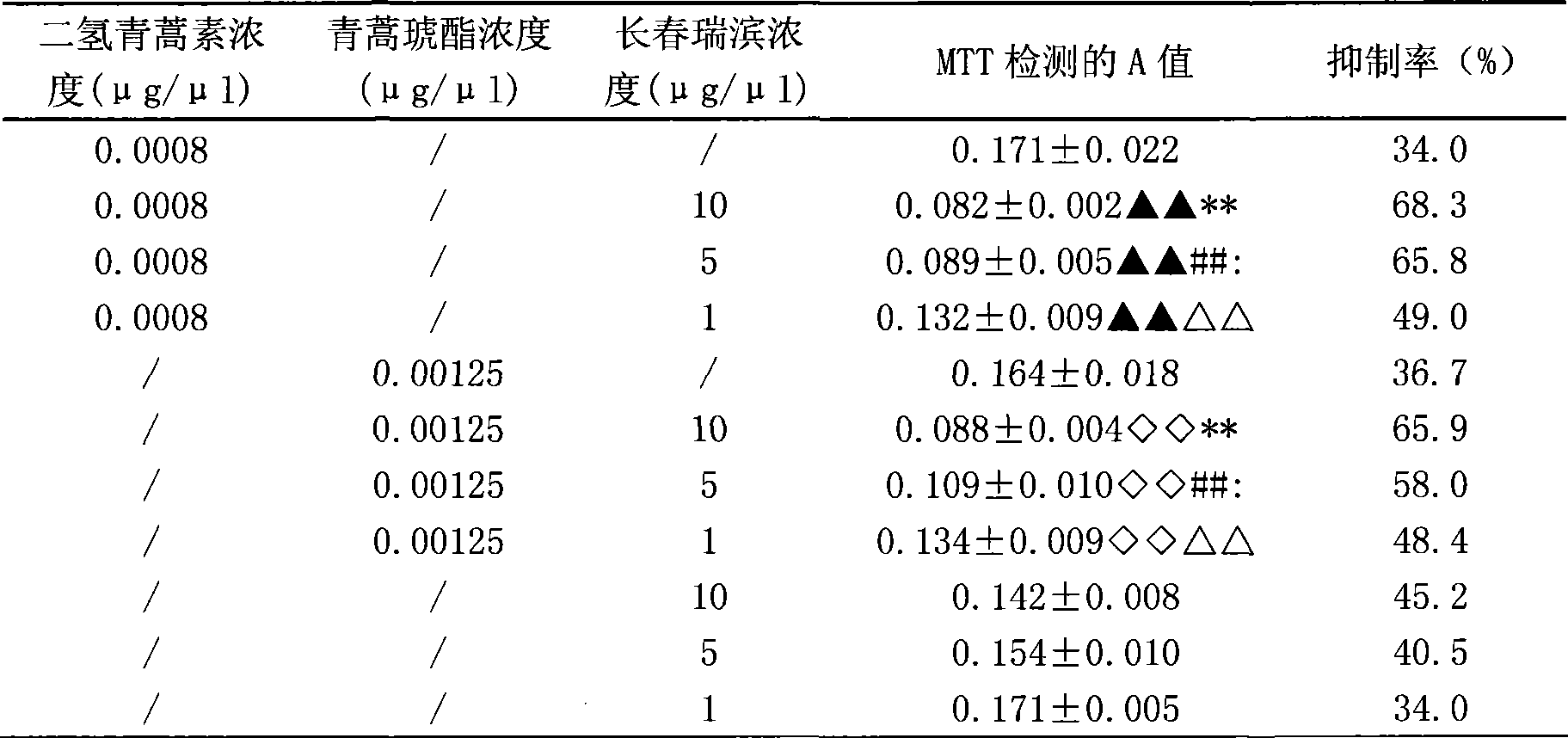
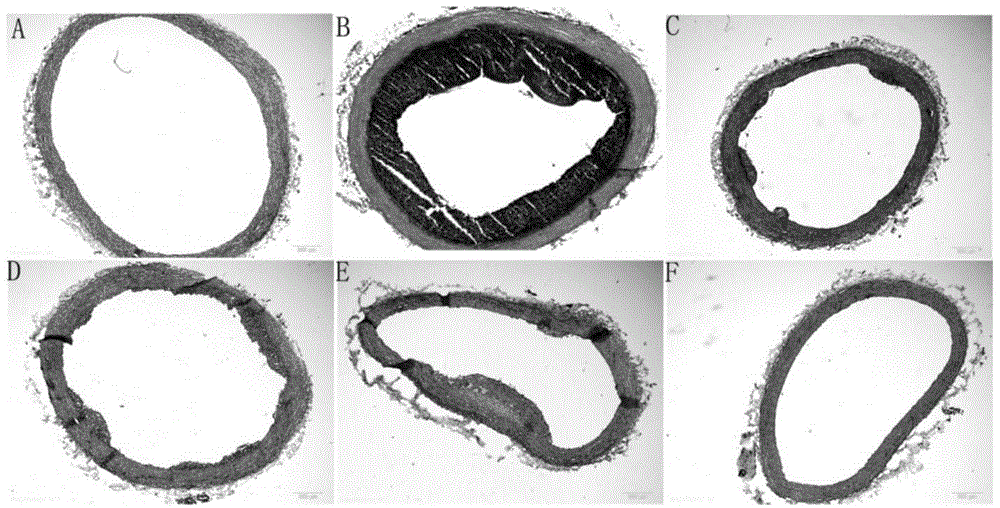
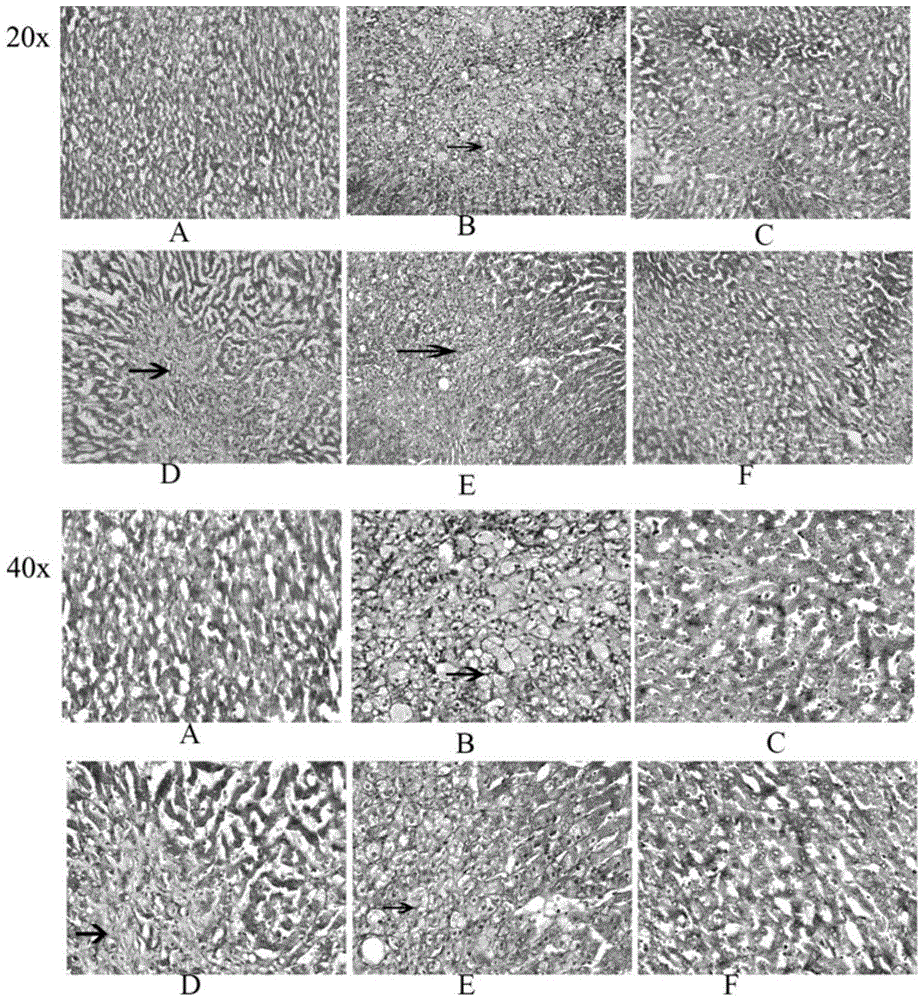
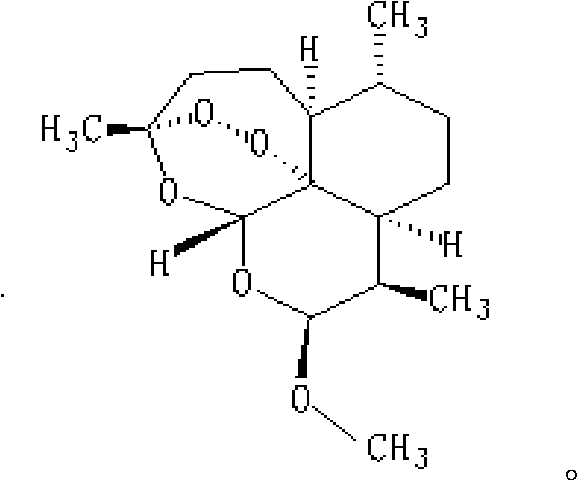
The aim of this study was to investigate the mechanisms of action of Dihydroarteannuin (DHA), a semi-synthesized agent from the starting material artemisinin extracted from the Chinese Traditional Herbs Artemisia annua, on ameliorating the symptoms of lupus on BXSB mice.
Medicinal compositon containing artemisine extract for treating rheumatoid arthritis and immunologic disease
InactiveCN1561994ASafe and effective treatmentOrganic active ingredientsAntipyreticDiseaseImmunologic disorders
A composite medicine for treating the umatoid arthritis and autoimmune disease contains the sweet worm wood herb's extract (dihydroarteannuin, artesumate, artemether, etc).
Owner:齐岩 +1
Combined application of artemisinin and its derivative and antibiotic medicine
InactiveCN101020056AIncrease useHigh antibacterial efficacyAntibacterial agentsHeterocyclic compound active ingredientsAntimicrobial drugMedicine
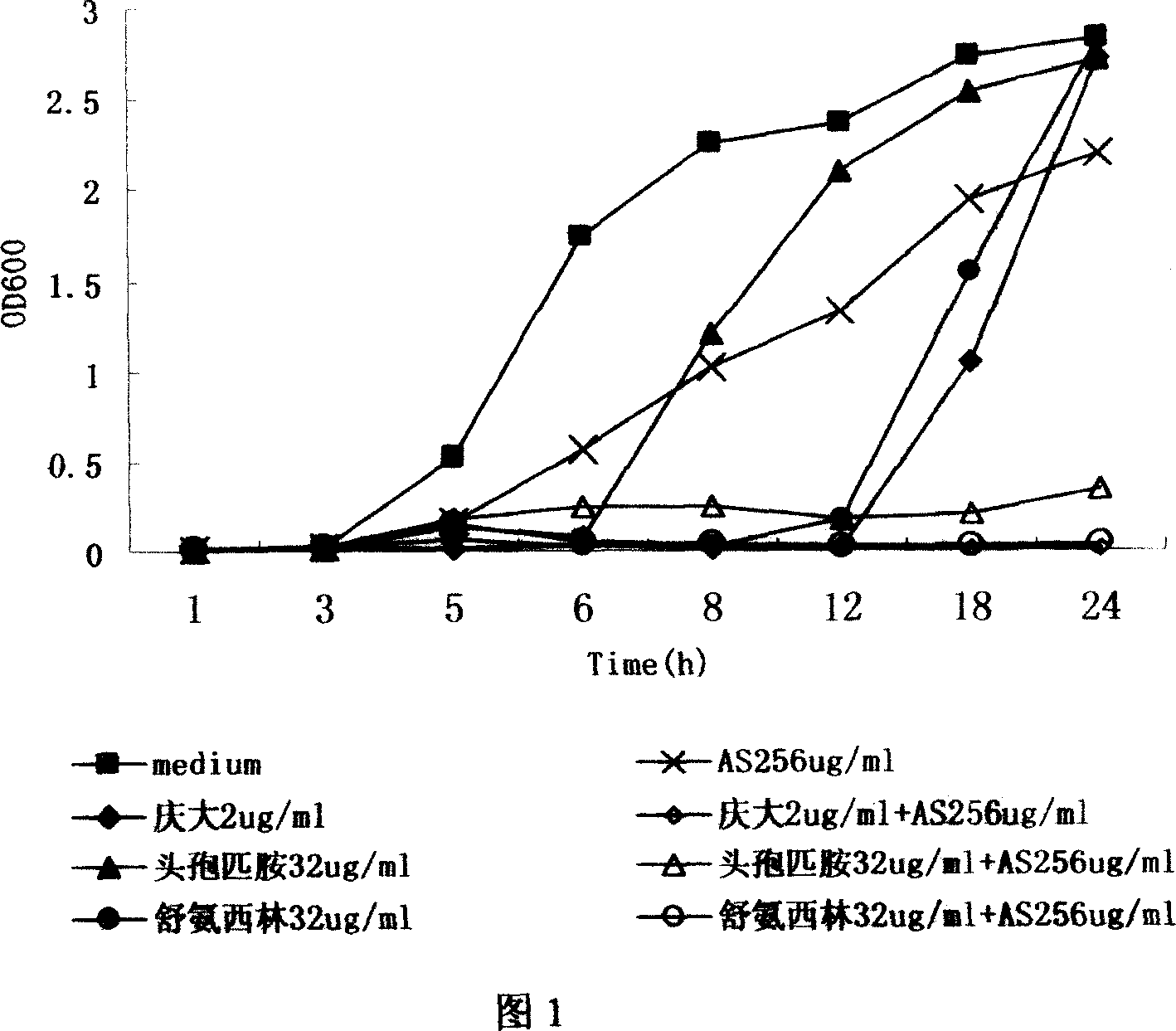
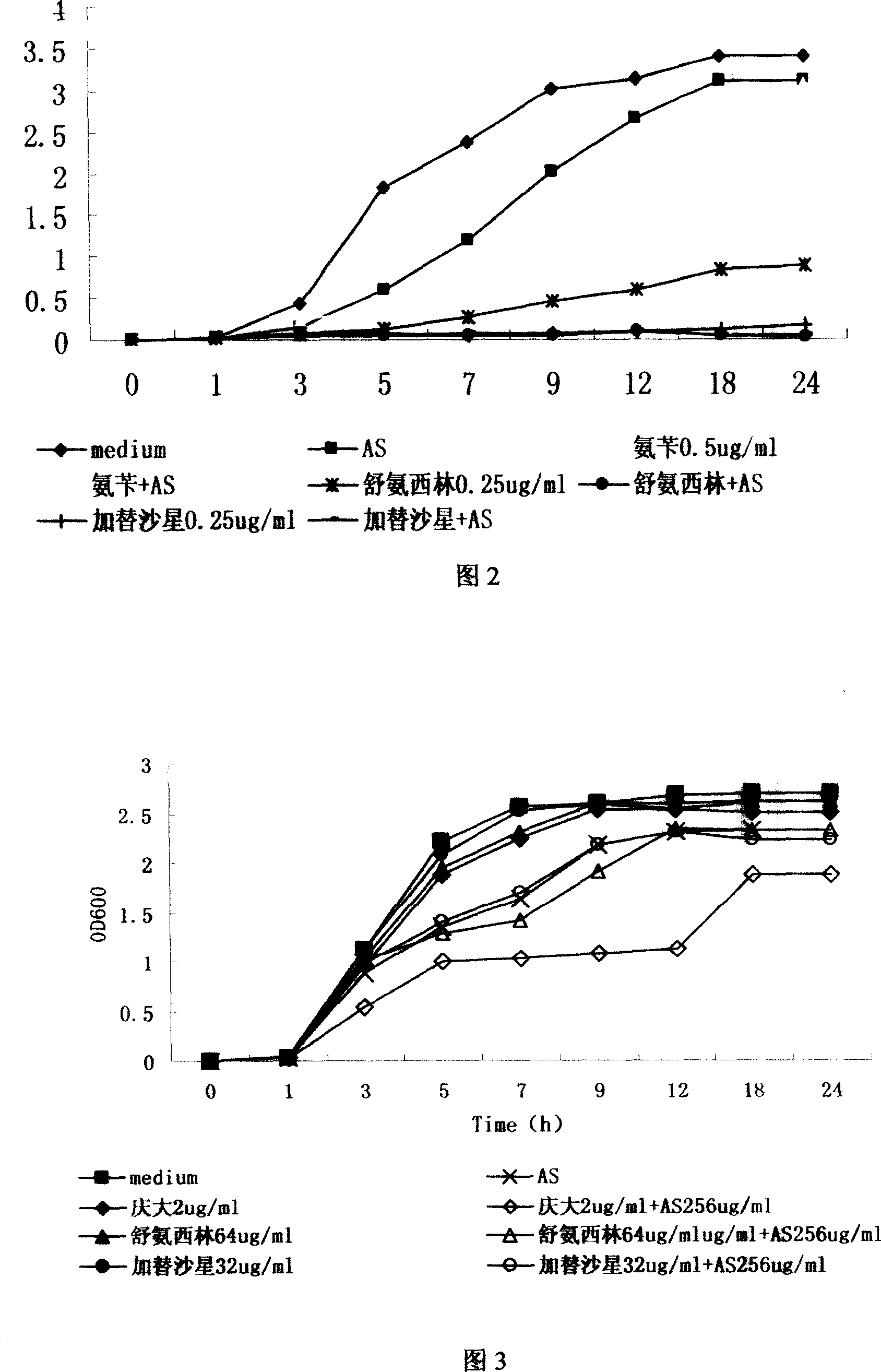
The present invention discloses the new use of available antimalarial arteannuin and its derivatives dihydro arteannuin, antiannuic methyl ether, antiannuic ethyl ether and antiannuic amber. Arteannuin and its derivatives are used through combination with antibiotic medicine to inhibit bacterial growth and enhance the antibiotic effect of available antibiotic medicine. Especially, antiannuic amber is applied in preventing and treating bacterial infection diseases except being used as antimalarial.
Owner:ARMY MEDICAL UNIV
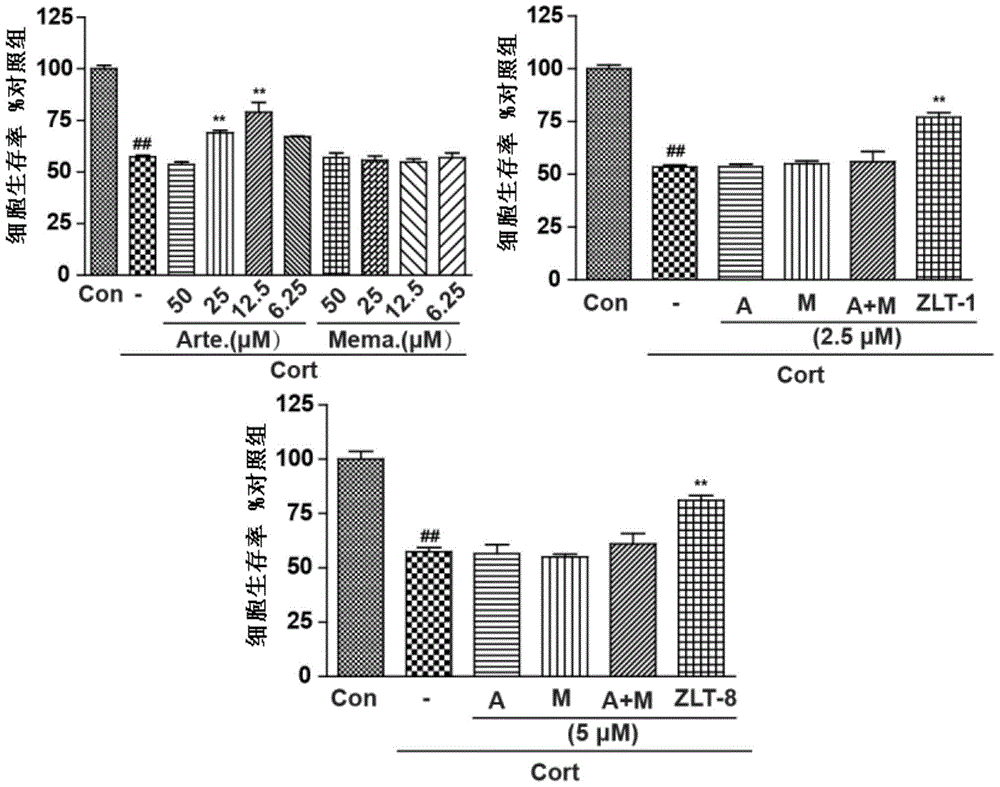
Dihydroarteannuin-memantine diad compounds, and synthesis method and application thereof
ActiveCN105732654ASimple preparation processSignificant effectOrganic active ingredientsNervous disorderMemantine HydrochlorideTreatment effect
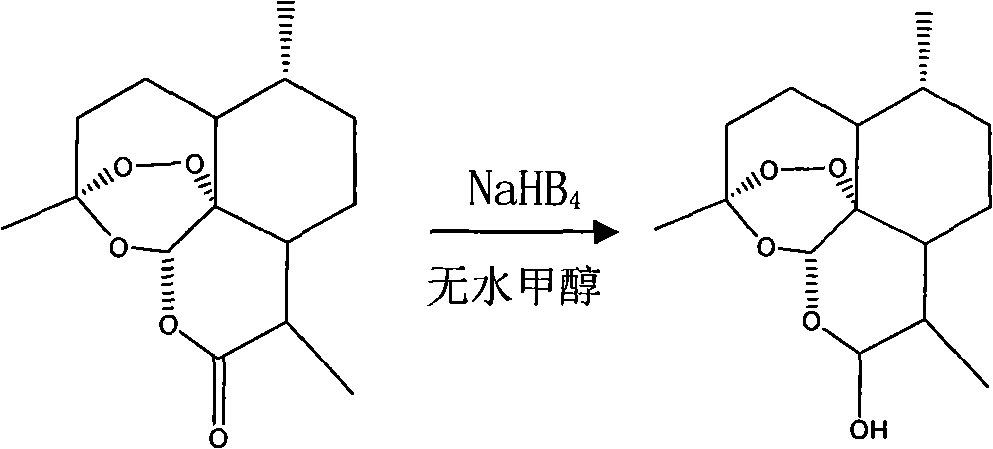
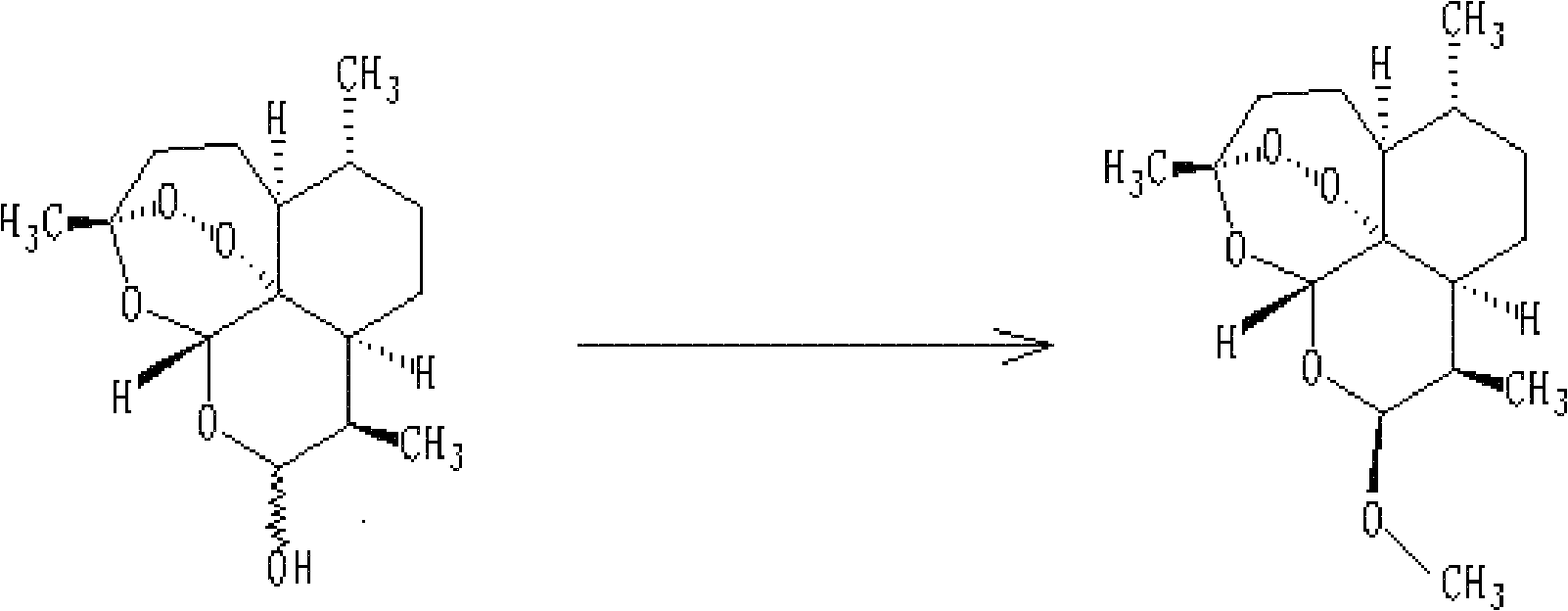
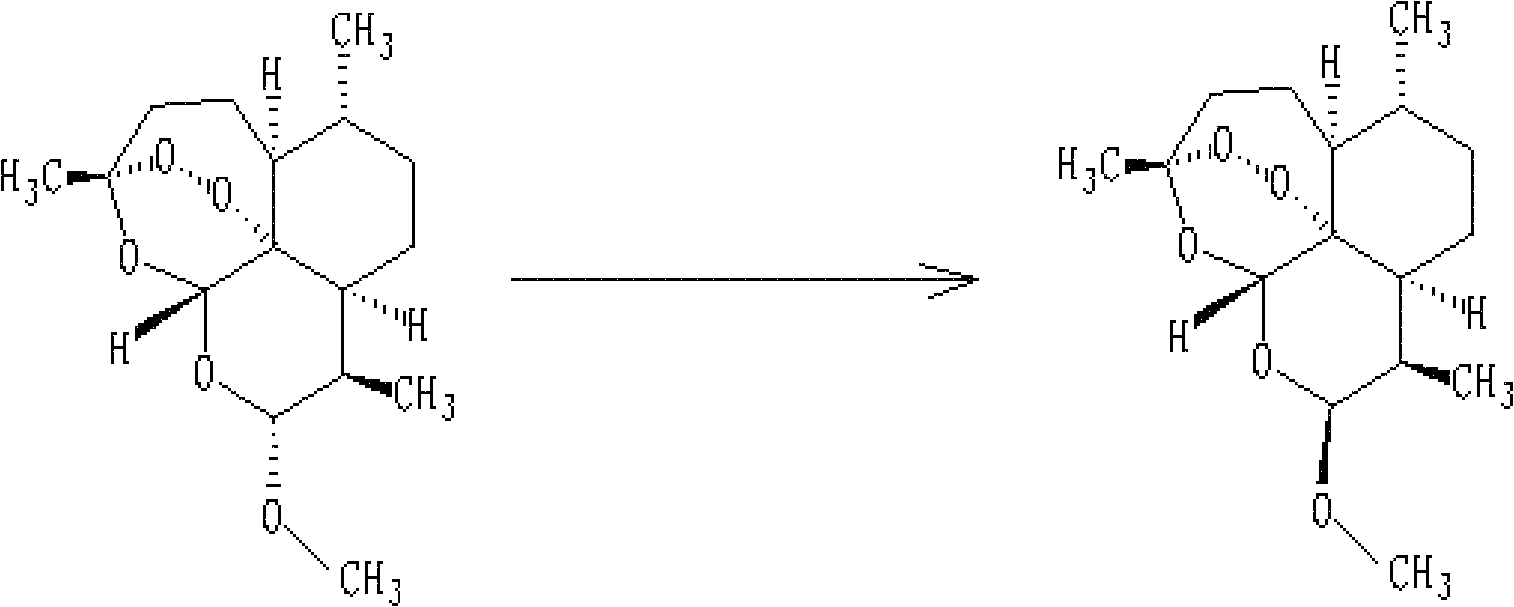
The invention discloses dihydroarteannuin-memantine diad compounds, and a synthesis method and application thereof. The structure of the compounds is disclosed as Formula I. The synthesis method comprises the following steps: reducing arteannuin to obtain dihydroarteannuin, carrying out acetalation reaction on the dihydroarteannuin and 2-bromoethanol under the catalytic action of Lewis acid, and carrying out reaction on the acetalation reaction product and memantine hydrochloride to obtain the dihydroarteannuin-memantine diad compounds. The compounds are reported for the first time, have an therapeutic effect on neurodegenerative diseases, and can be used for preparing drugs for treating neurodegenerative diseases. Compared with other prior arts, the compounds disclosed by the invention have the advantages of simple preparation technique and better curative effect than memantine.
Owner:JINAN UNIVERSITY
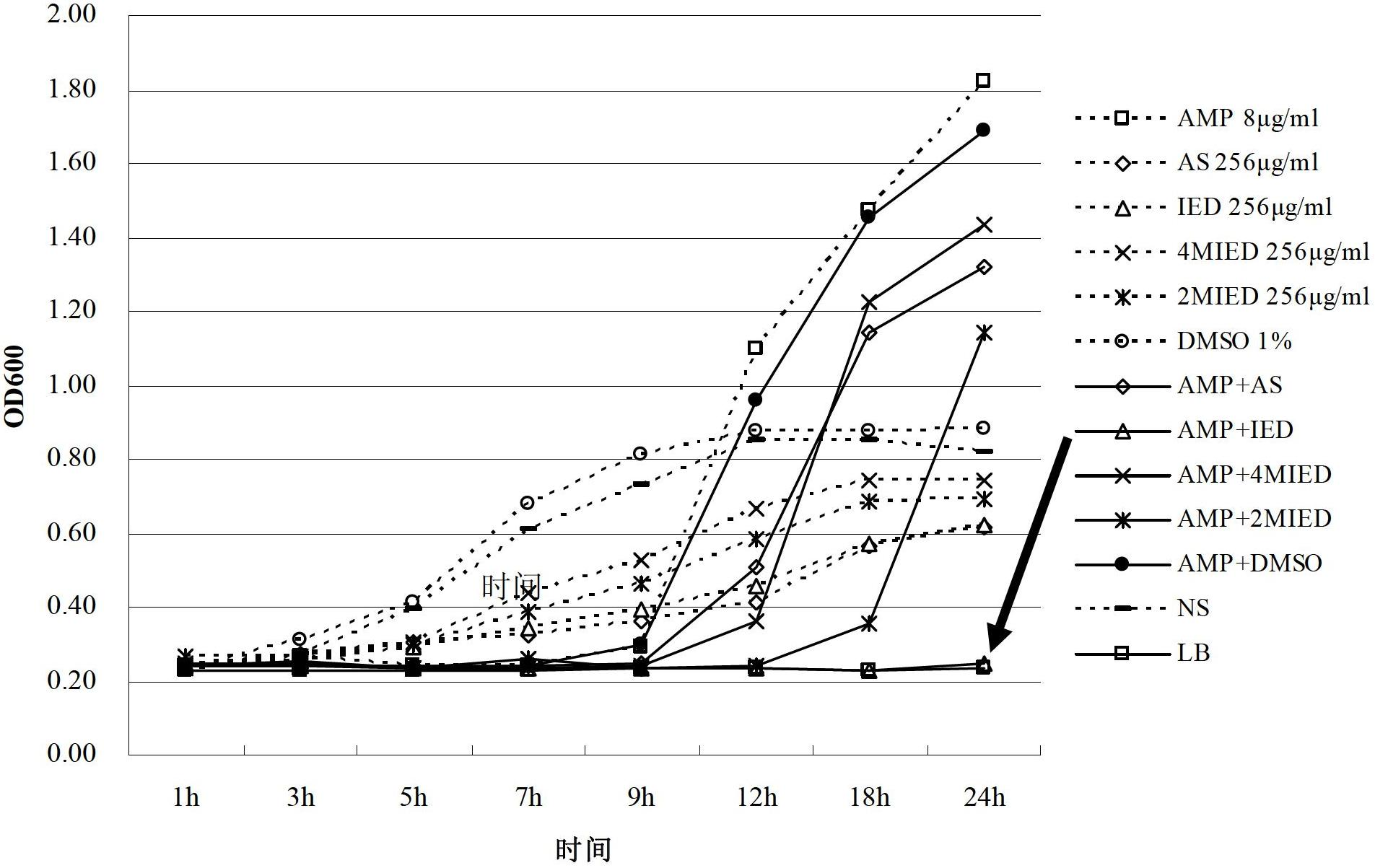
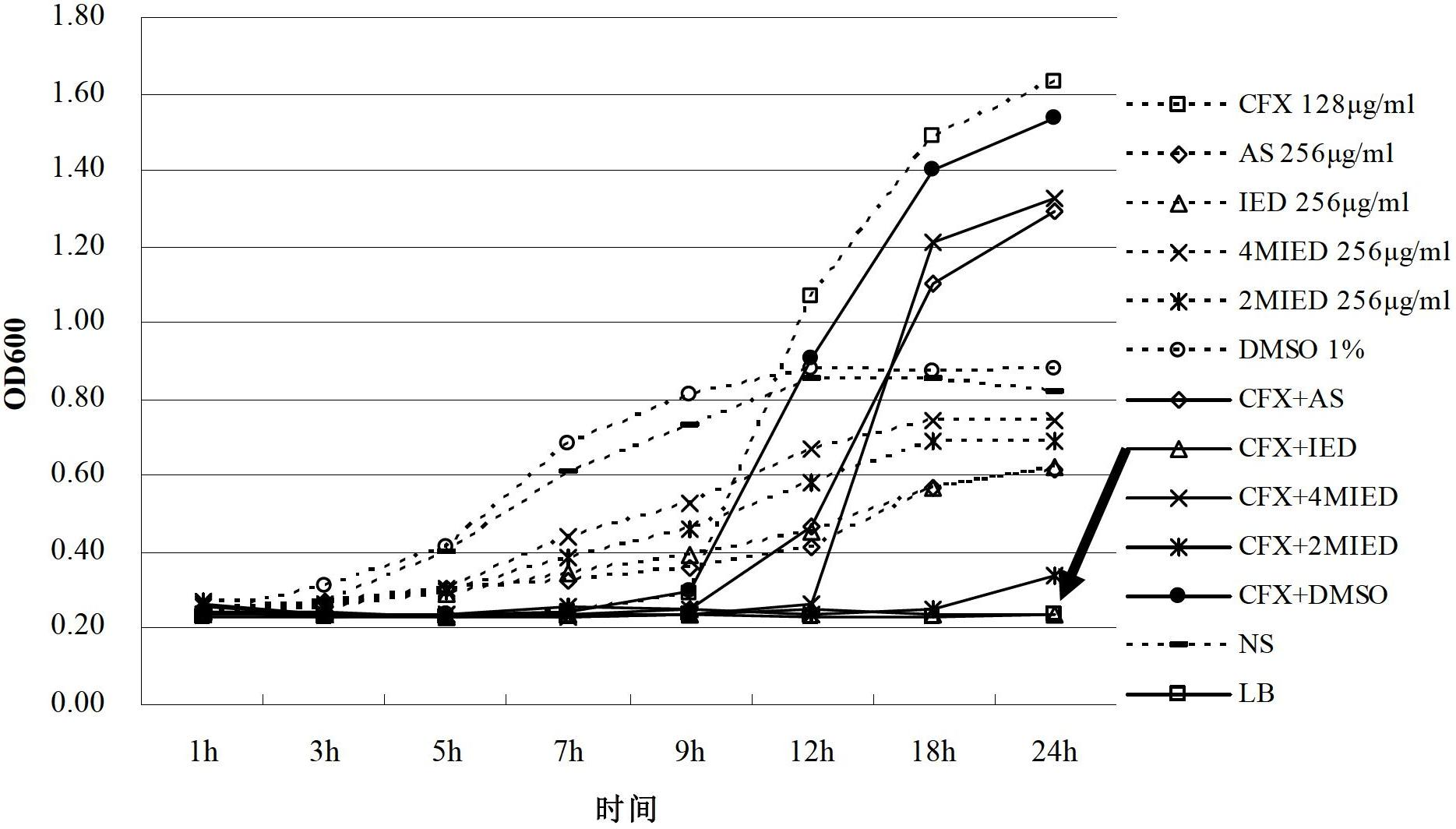
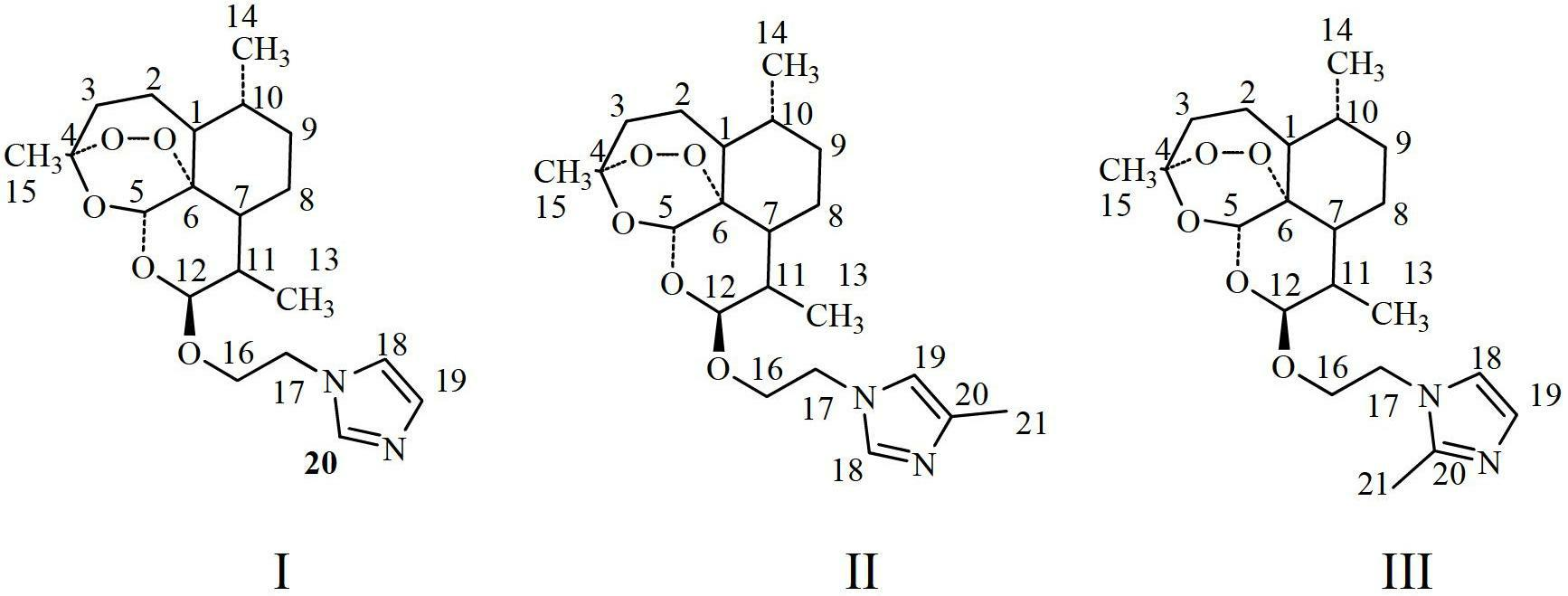
Dihydroarteannuin derivatives and application thereof
InactiveCN102675337AHigh antibacterial efficacyReverse drug resistanceAntibacterial agentsOrganic chemistryEscherichia coliBeta lactam antibiotic
The invention relates to synergistic dihydroarteannuin oxyethylimidazole derivatives and application thereof. The dihydroarteannuin oxyethylimidazole derivatives have the structure disclosed as one of Formulae I-III, and can be used for preparing an antimicrobial synergist for antibiotics. The dihydroarteannuin derivatives can inhibit growth of multidrug-resistant Escherichia coli when being used independently or combined with beta-lactam antibiotics, reverse the drug resistance of the antibacterial drug to bacteria, and enhance the antimicrobial efficacy of antibiotics.
Owner:ARMY MEDICAL UNIV
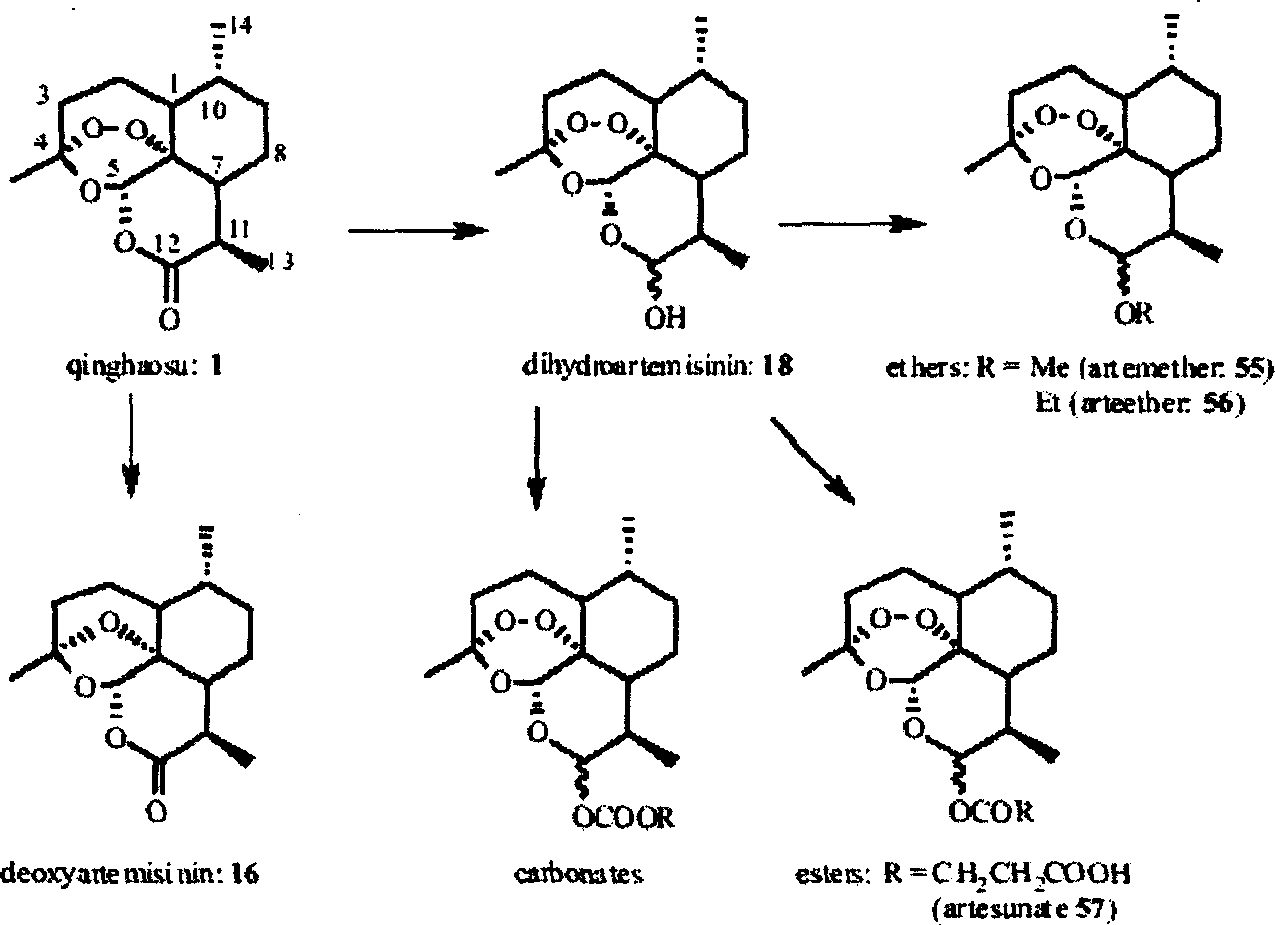
[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof
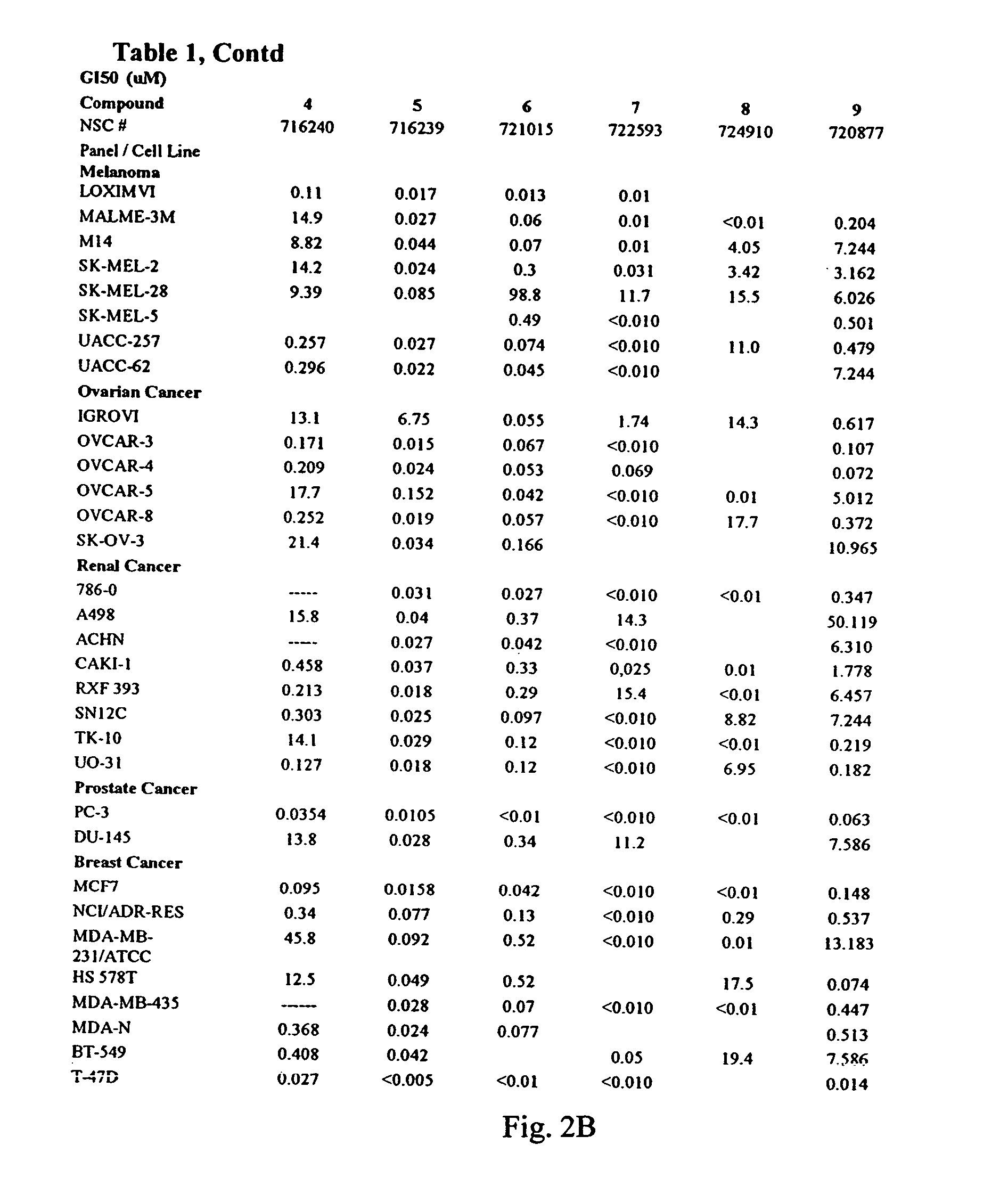
The invention relates to [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances and provides a structure, a preparation method and application of a novel artemisinine 10-locus derivative. The structural formula of the [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substance is shown in a formula I. The invention also relates to pharmaceutically-acceptable slats, a solvate, an optical isomer or a polymorphic substance of the [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances and a medicinal composition by taking the compounds as active components. As novel antimalarial agents, anti-tumor agents and antifungal agents, the compounds can be used for treating or preventing malaria, mycotic infection, malignant tumor and the like. The compounds can be prepared by reacting dihydroartemisinine as an initial raw material with trifluoroacetic anhydride / triethylamine to obtain 10(R)-trifluoro-acetoxyl-9,10-dihydroartemisinine, directly reacting the 10(R)-trifluoro-acetoxyl-9,10-dihydroartemisinine with hydroxy benzaldehyde without separation to obtain (10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde, and reacting the (10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde with the substituted amino (sulfur) urea compounds in acid catalysts and alcohol solvents to obtain target compounds.
Owner:SHENYANG PHARMA UNIVERSITY
Application of dihydroartemisinin to preparation of tumor cell autophagy induction medicament
InactiveCN102038678AGrowth inhibitionInhibition of newbornsOrganic active ingredientsAntineoplastic agentsPharmaceutical formulationWilms' tumor
The invention provides application of dihydroartemisinin to preparation of a tumor cell autophagy induction medicament. The molecular formula of the dihydroartemisinin is C15H24O5. The medicinal preparation comprises a preparation-allowable medicinal excipient or carrier. The excipient is in the form of a solid preparation. The medicinal preparation provided by the invention can be used as a mitochondrion targeting autophagy inductor, which has a specific anti-tumor effect and can provide a therapeutic medicament for overcoming medicament resistance and improving prognosis for treatment of tumors. The effects and the mechanisms of effective monomer components in a traditional Chinese medicine are research and described under the background of a cell autophagy theory; and an important basis is provided for the development of new theory of the traditional Chinese medicine and new acting target of the medicament, and a new research direction for developing the theory of the traditional Chinese medicine is provided.
Owner:ZHEJIANG UNIV
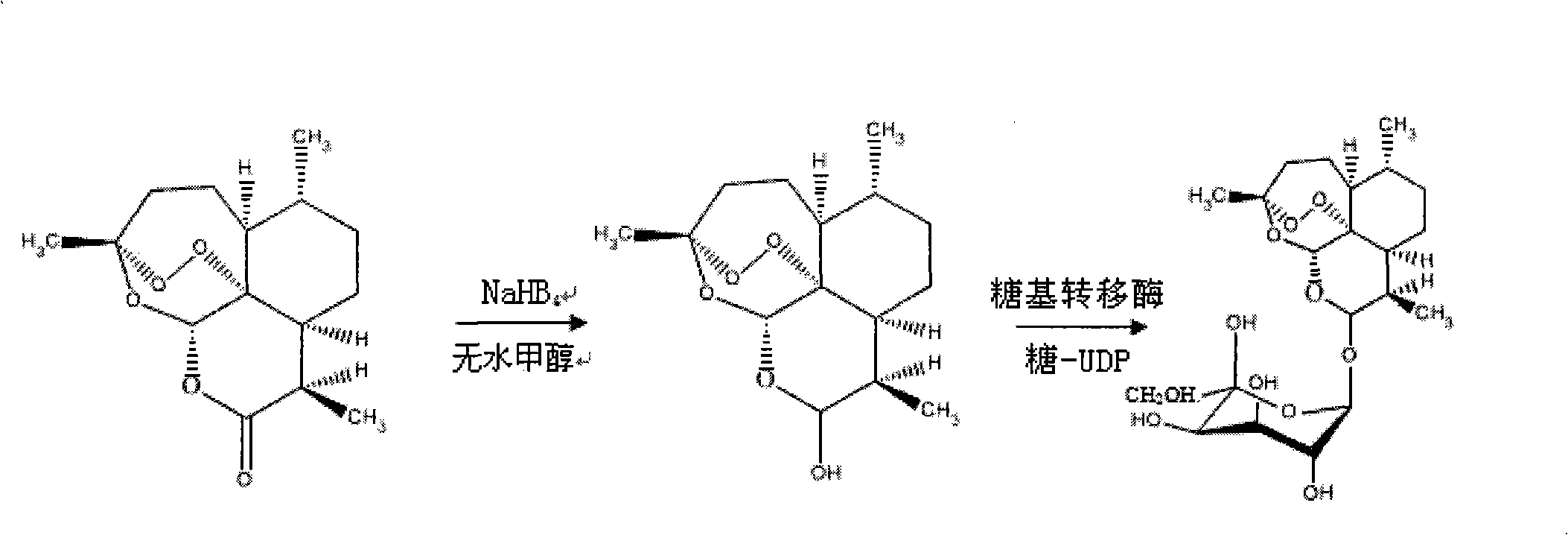
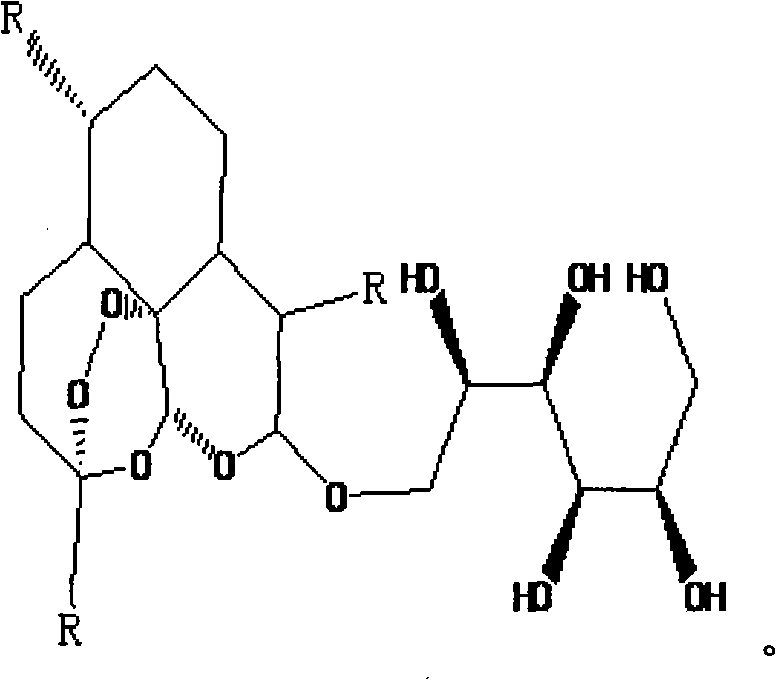
Galactose-artemisinin and method for preparing same
InactiveCN101307082AImprove biological activityPlay a synergistic roleOrganic active ingredientsSugar derivativesUDP galactoseSide effect
The invention relates to a galactose-arteannuin and a preparation method for the same. The preparation method comprises the following: (1) a step of preparing dihydro-arteannuin; (2) a step of preparing UDP-galactose; (3) a step of preparing the galactose-arteannuin. The invention adopts glycosyltransferase to catalyze the glycosylation reaction of the arteannuin with gentle conditions during a synthetic process, convenient operation, high product purity and higher yield. An elementary cell experiment verifies that: the galactose-arteannuin has better performance of inhibiting the activity of a tumor cell Hela than the parent substance of the arteannuin without any toxic and side effect on normal cells. The galactose-arteannuin prepared through the method is easy to prepare with a good pharmaceutical prospect.
Owner:CHONGQING UNIV
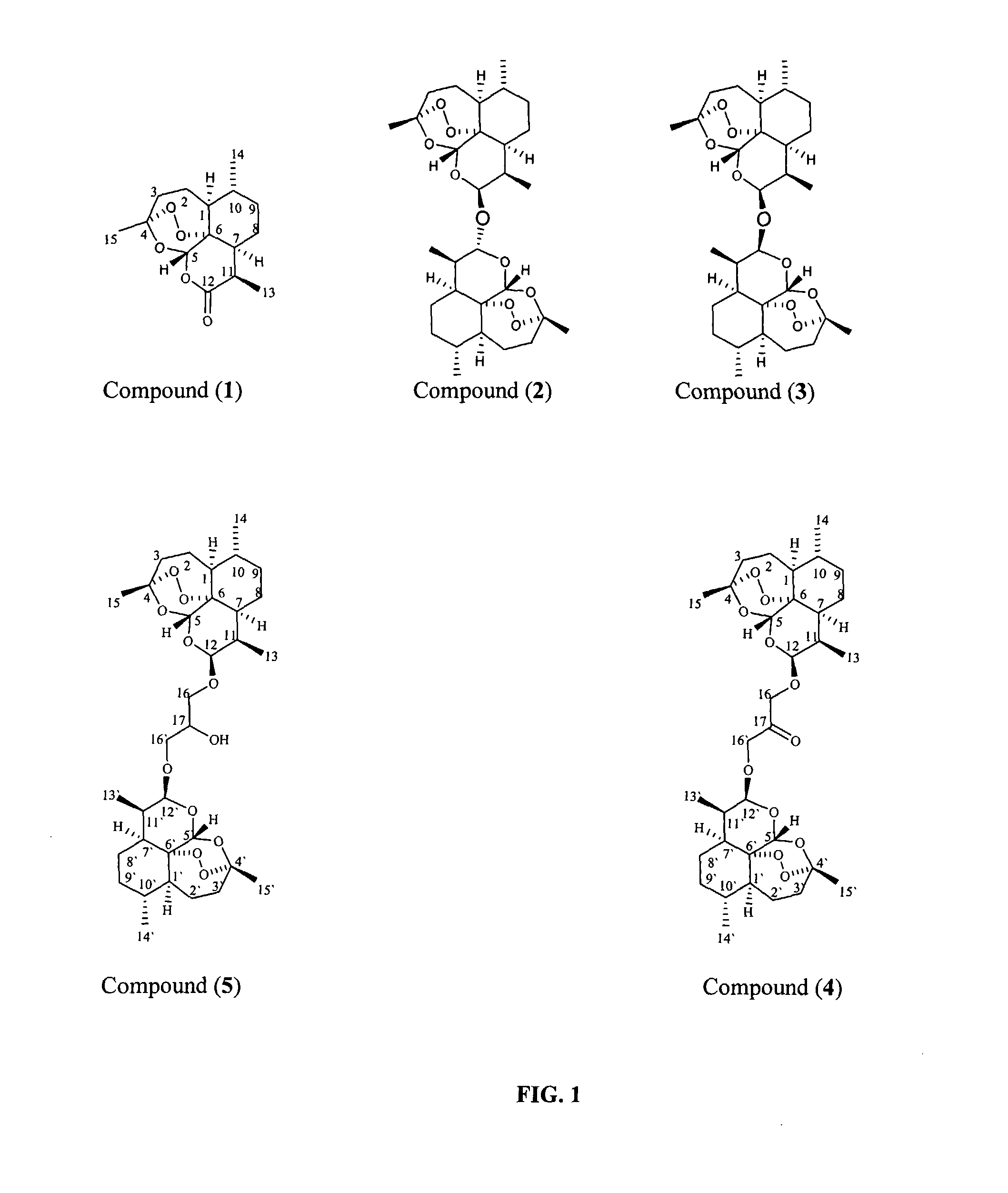
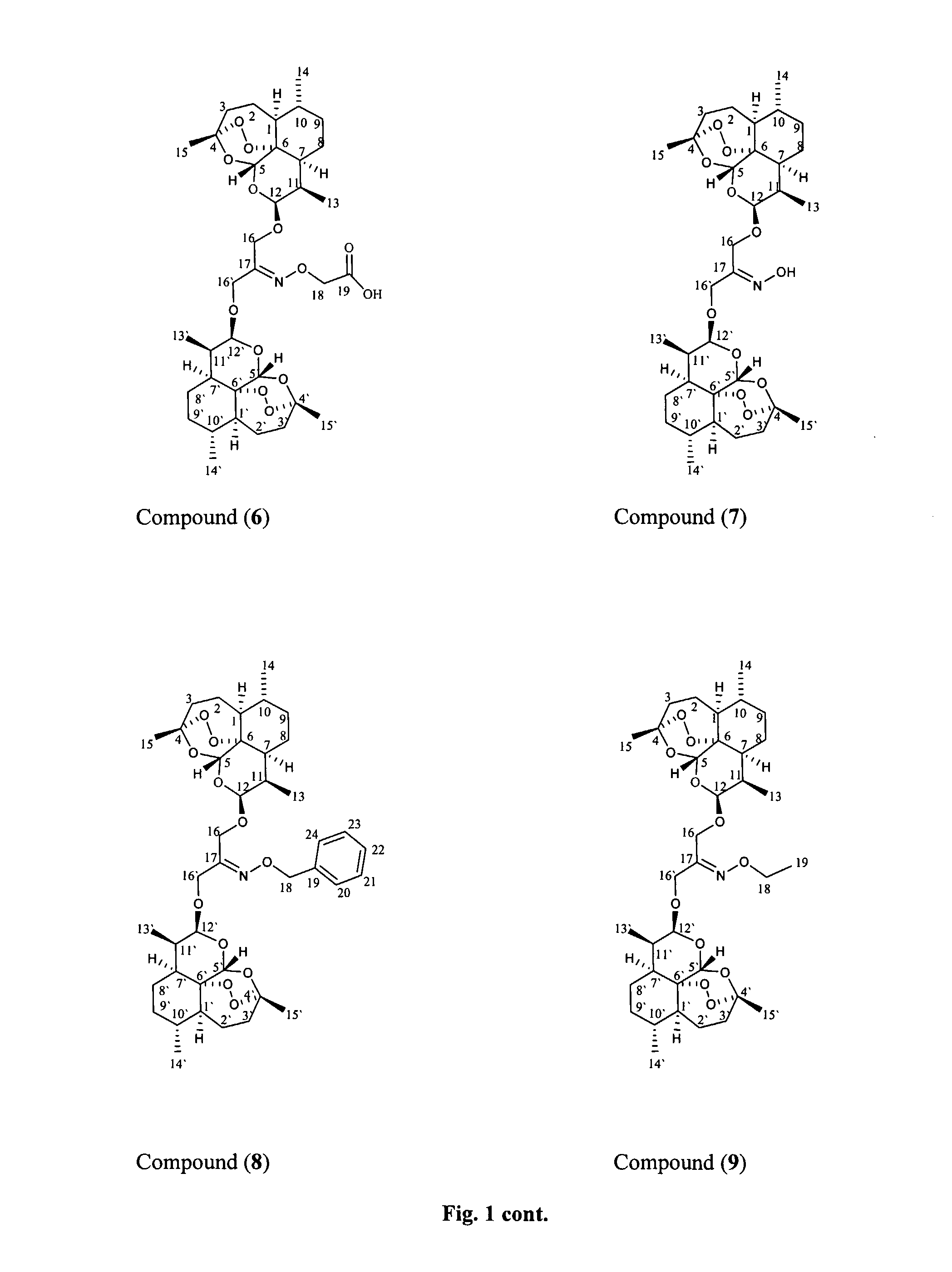
Dihydroartemisinin and dihydroartemisitene dimers as anti-cancer and anti-infective agents
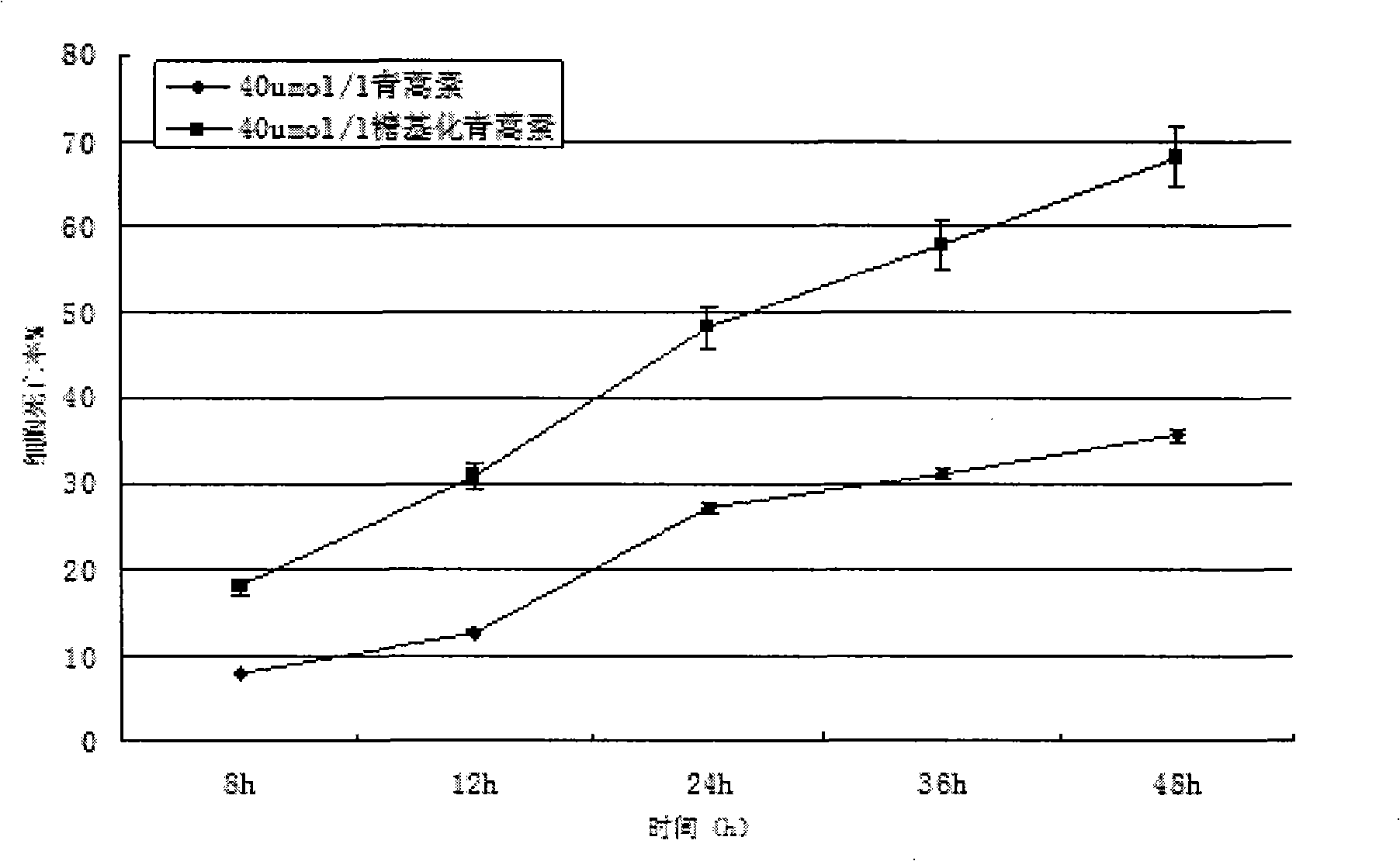
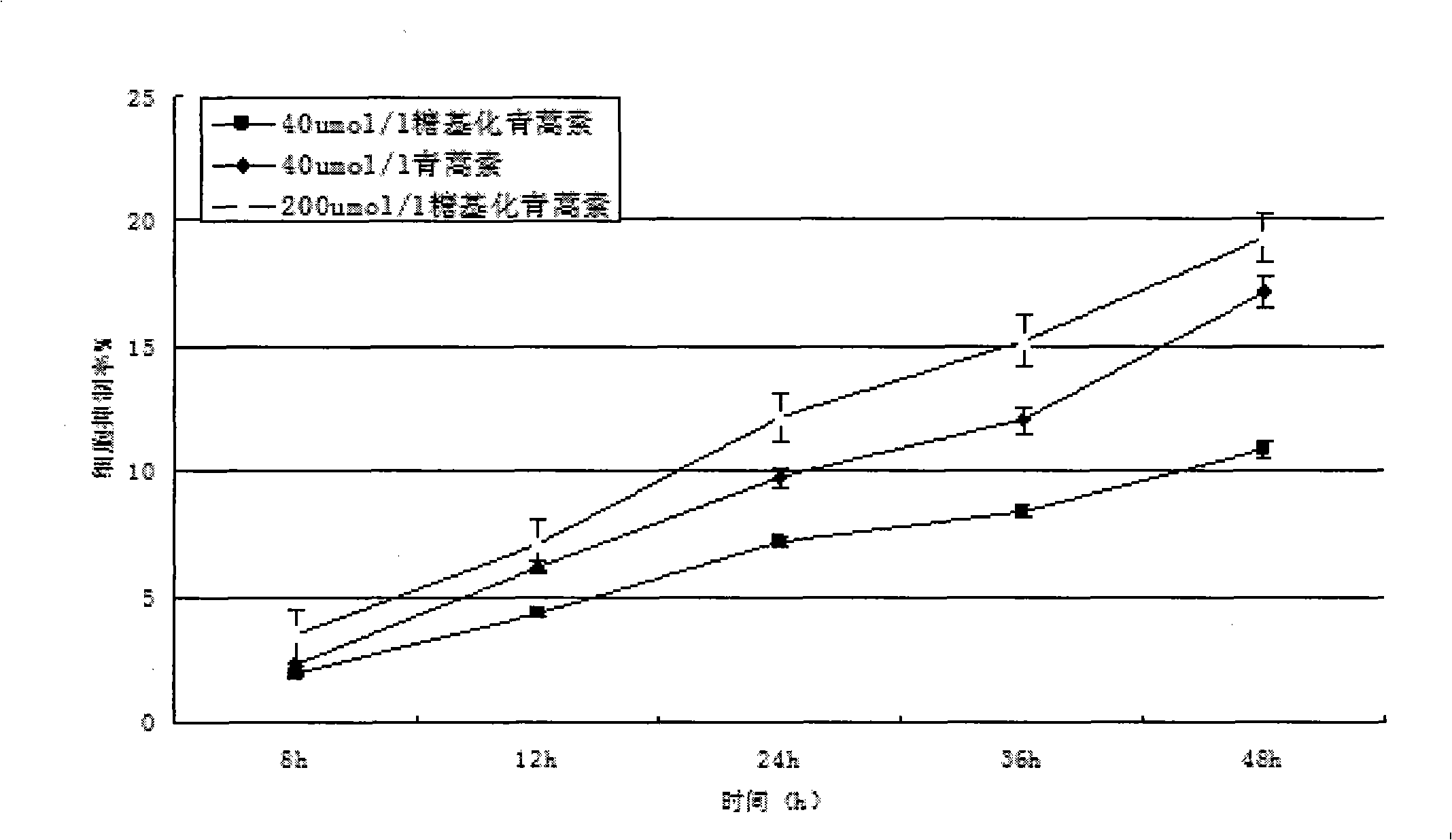
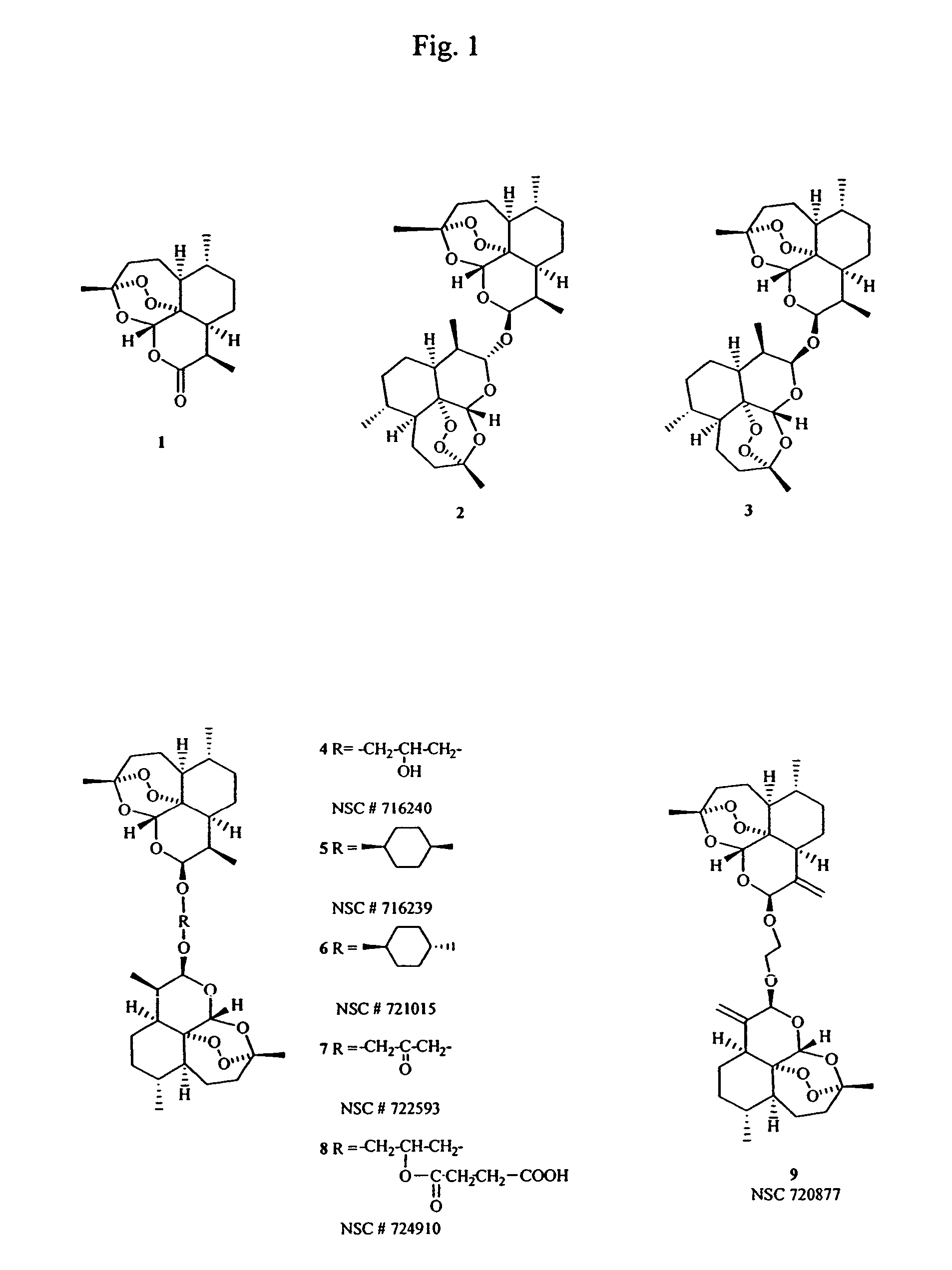
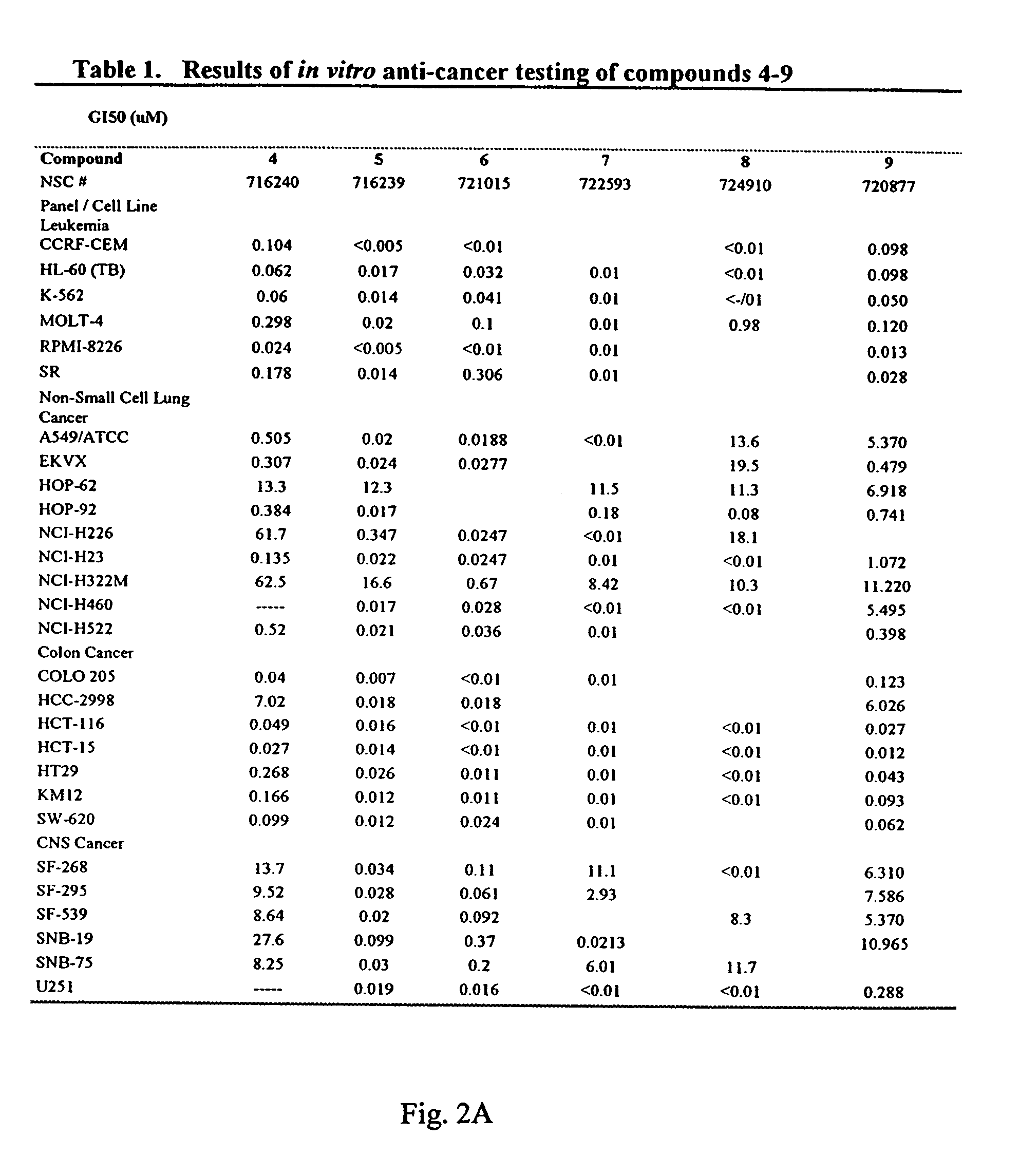
This invention comprises compositions containing dihydroartemisinin and dihydroartemisitene dimers with activity as anticancer agents and anti-protozal, including anti-malarial and anti-leishmanial properties. This invention also describes methods of preparation of these compositions and methods of use of such compositions for the treatment of cancer, and protozoal infections, including malaria, or leishmaniasis.The compounds of this invention represent a potential new class of anti-tumor agents, one that has shown promising activity against solid tumors, and with a pattern of selectivity that suggests a possible new mechanism of action.
Owner:ELSOHLY LAB
Application of dihydroartemisinin in reinforcing chemotherapy medicine antitumor curative effect
InactiveCN101125140AGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismPharmacometricsTumor chemotherapy
The present invention provides an application of dihydroartemisinin in the preparation of the drugs to enhance the anti-tumor efficacy of the chemotherapeutic drugs. The pharmaceutical preparation contains preparation allowable pharmaceutical excipients or carriers. The form of the preparation is solid preparation. The pharmaceutical preparation provided by the present invention can enhance the anti-tumor efficacy of the chemotherapeutic drugs and can be used in the adjuvant therapy during the tumor chemotherapy process. The present invention takes the theory that the angiogenesis inhibitor can change the micro-environment of tumor as the background, studies and clarifies the function and the mechanism that the effective monomer in the traditional Chinese medicine can enhance the anti-tumor efficacy of the chemotherapy drugs, provides a pharmacological basis for the development of the new usages of the artemisinin drugs and provides an important basis for the development of the new clinical values of the angiogenesis inhibitor, so the present invention is a new research direction for the development of the theory of traditional Chinese medicine.
Owner:ZHEJIANG UNIV
Abrotine, its derivative dihydro-abrotine, artemether, arteether and arte sunate in use of pharmacy
InactiveCN1833644AEnhance pharmacological effectsSignificantly in vivoAntibacterial agentsHeterocyclic compound active ingredientsEscherichia coliBacteroides
An application of arteannuin and its derivatives (dihydroarteannuin, artemether, arteether and artesunate) in preparing the medicines for preventing and treating the sepsis caused by CpG-ODN and bacteria is disclosed.
Owner:ARMY MEDICAL UNIV
Composition of artemisinin derivative and vinorelbine, and use thereof
InactiveCN101380325AReduce dosageSynergisticOrganic active ingredientsAntineoplastic agentsAdjuvantOral medication
The invention relates to a composite of an artemisinin derivative and vinorelbine in the technical field of pharmaceuticals and the application thereof; wherein, the ratio of the artemisinin derivative (including dihydroartemisinin or artesunate) and the vinorelbine is 1:500 to 500:1. The composite can be applied to preparing antineoplastics and made into the dosage forms of injection administration and oral administration by adding pharmaceutically acceptable adjuvant carriers. The two effective components (the artemisinin derivative and the vinorelbine) of the composite not only have obvious synergistic effect and but also can be cooperatively applied so as to reduce the amount of the vinorelbine and lower the production cost. By adding the pharmaceutically acceptable adjuvant carriers to prepare the dosage forms of injection administration and oral administration, the composite can be applied to the anti-tumor field.
Owner:SHANGHAI JIAO TONG UNIV
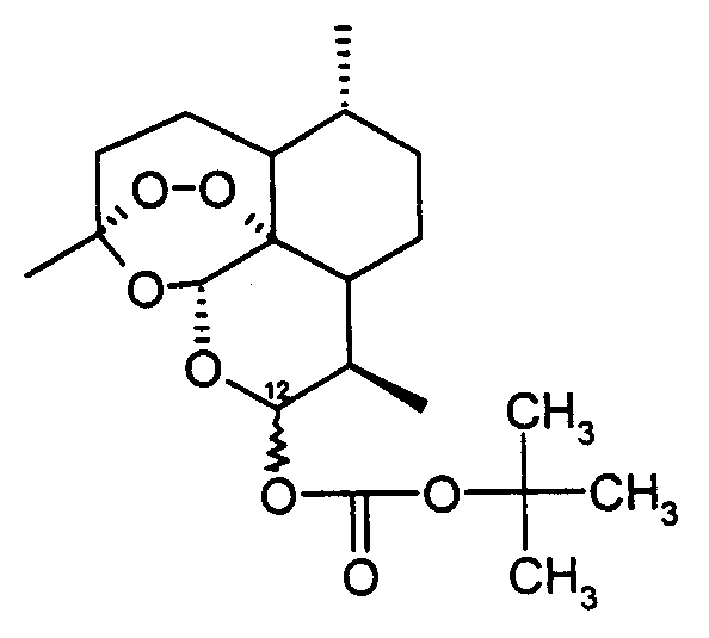
Tert-butoxy carbonyl dihydro artemisinin, preparation method and drug composition thereof
InactiveCN1405168AHigh activityLow toxicityOrganic active ingredientsOrganic chemistryDicarbonateTert-Butyloxycarbonyl protecting group
The invention provides a tert-butoxycarbonyl dihydroartemisine, which is characterized by that said invention provides its structural formula, and it is made up by using dihydroartemisine as initiation raw material, and making it and double tertiary butyl dicarbonate implement acidation reactino in organic solvent. The invented mecicine composition for resisting parasitic disease contains tert-butoxycarbonyl dihydroartemisine with therapeutic effective dose and pharmaceutically-acceptable carrier. Said invented product is high in therapeutical effect and low in toxicity, can be used for preventing and curing the parasitic diseases of schistosomiasis and malaria, etc.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
A method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma
InactiveCN103940918AHigh purityImprove concentrationComponent separationSolid phase extractionSolvent
The invention discloses a method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma. The method comprises steps of: extracting a blood plasma sample with a solvent, processing the sample liquid with a solid phase extraction technology, adding the sample liquid into a small processed solid-phase extraction column, washing the column with acetic acid and a methanol-acetic acid solution, eluting with ethyl acetate and 1-chorobutane, collecting the eluate, blowing the eluate to dry with nitrogen, dissolving residue with methanol so as to obtain a sample solution to be tested, and accurately measuring the content of the artesunate and the content of the dihydroartemisinin in the sheep blood plasma by utilization of HPLC-MS / MS. The lowest detectable limit of the artesunate is 0.1 ng*mL<-1>. The lowest detectable limit of the dihydroartemisinin is 1 ng*mL<-1>. The method reduces influences of impurities, enriches the sample concentration and is high in specificity and good in separation effect. Linearity, stability, reproducibility, and recovery tests of the method satisfy good technical requirements. The method lays methodology foundations for research of pharmacokinetics and bioequivalence of the medicine inside animals.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Application of artemisinin-typed compound in preparation of drug for treating and preventing hyperlipidemia
ActiveCN104398505AWide variety of sourcesLow priceOrganic active ingredientsMetabolism disorderPharmacologic actionSecondary hyperlipidemia
The invention discloses an application of an artemisinin-typed compound in preparation of a drug for treating and preventing hyperlipidemia. The artemisinin-typed compound is artemisinin, artesunate, dihydroartemisinin or artemether. The artemisinin-typed compound can be prepared into any pharmaceutically-acceptable preparation. The invention develops new medical applications of the artemisinin-typed compound. The application overcomes defect of a drug for treating and preventing the hyperlipidemia in the prior art. The drug can achieve a treatment effect from inflammatory resistance and blood fat reducing. Meanwhile, the artemisinin-typed compound is a drug which is firstly developed in our country, which means that our country is a main producing country, so that the artemisinin-typed compound is wide in sources and is low in price. In addition, the artemisinin-typed compound is strong in pharmacologic action, is high in safety, and provides an economical and safe drug for patients suffered from cardiovascular diseases.
Owner:SHANGHAI JIAO TONG UNIV
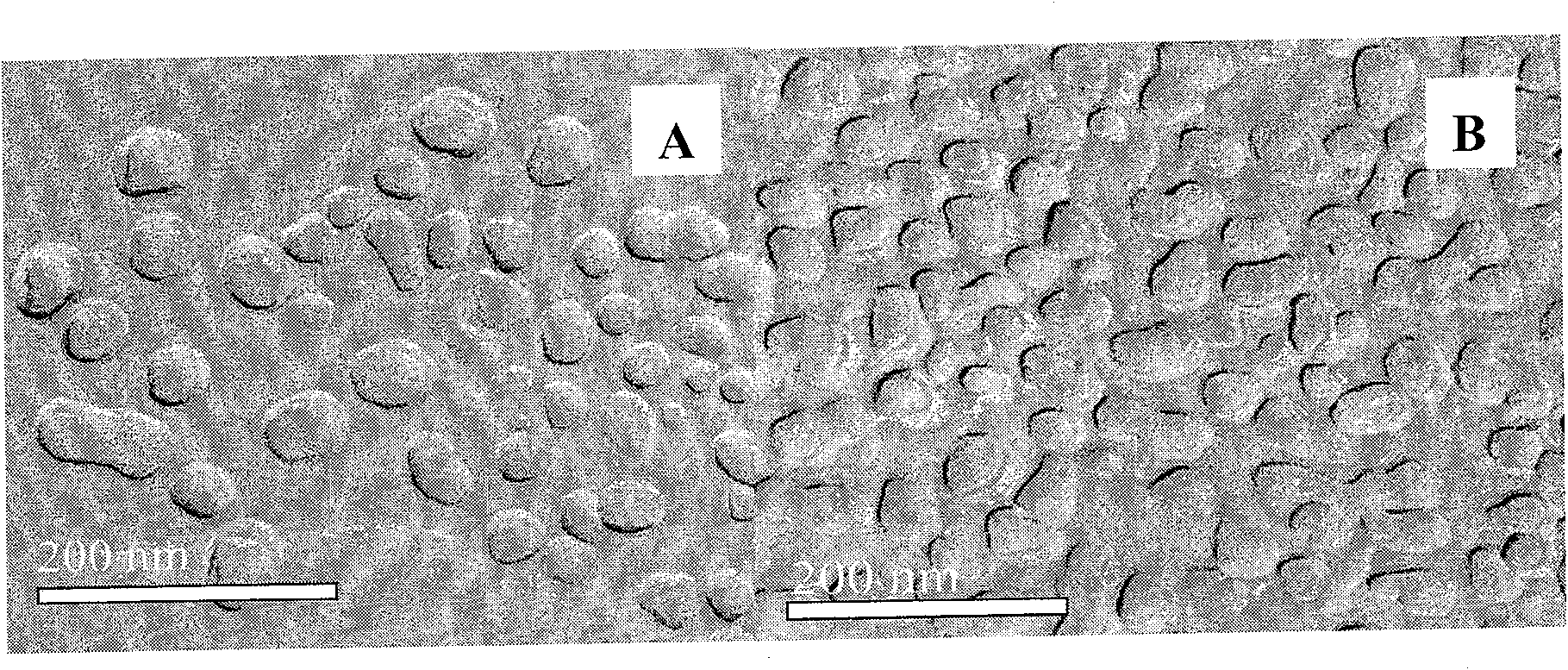
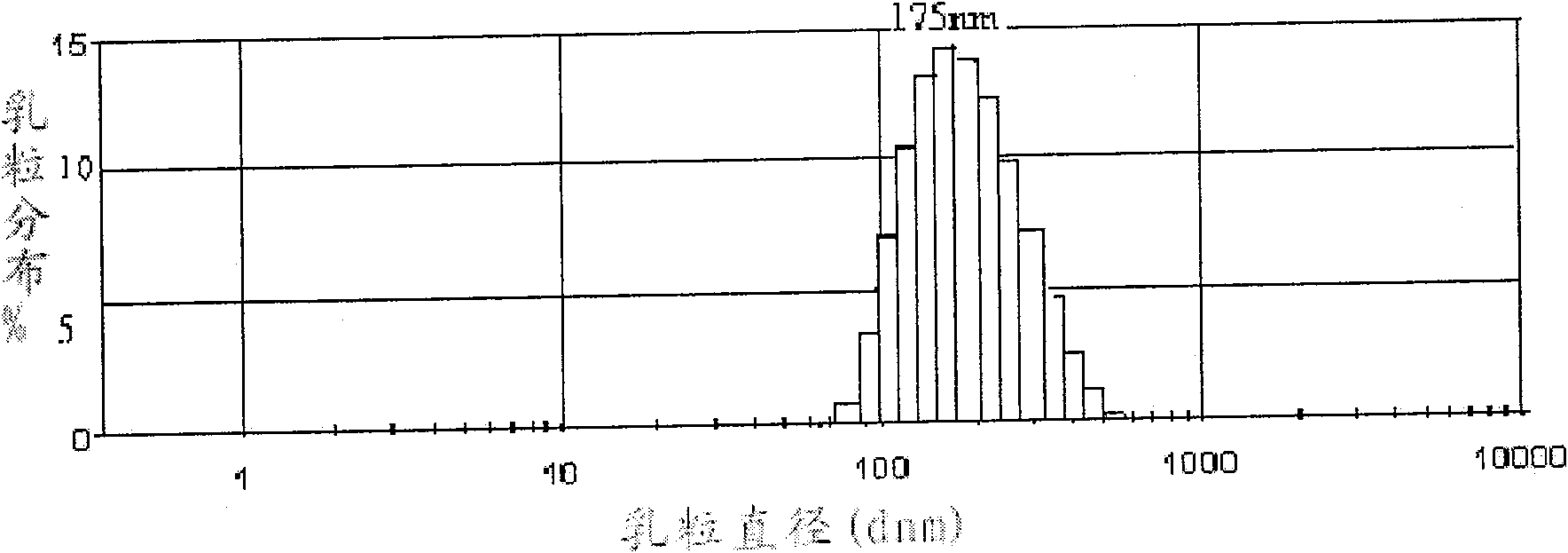
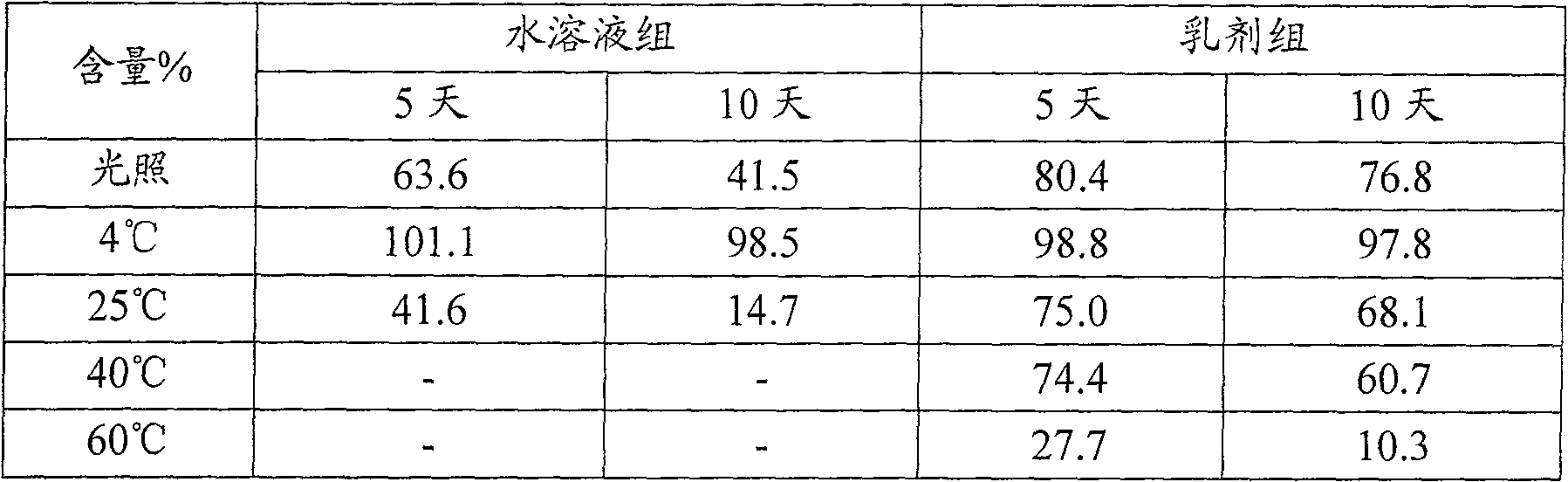
Fatty artemisinin emulsion and its prepn and application
InactiveCN1771909AExtended half-lifeImprove targetingOrganic active ingredientsEmulsion deliveryMedicineDihydroartemisinin
The present invention discloses one kind of fatty artemisinin emulsion and its preparation and application. The fatty artemisinin emulsion includes oil for injection, smulsifier, solubilizer and isotonizer, and has artemisinin or dihydroartemisinin well dissolved in oil and coated in emulsified oil phase, so that it has high stability, strengthened medicinal effect, slow releasing and targeting administration effect, prolonged medicine residence time in blood and raised bioavailability.
Owner:裴蕾
Water-soluble arteannuin derivative and preparation method thereof
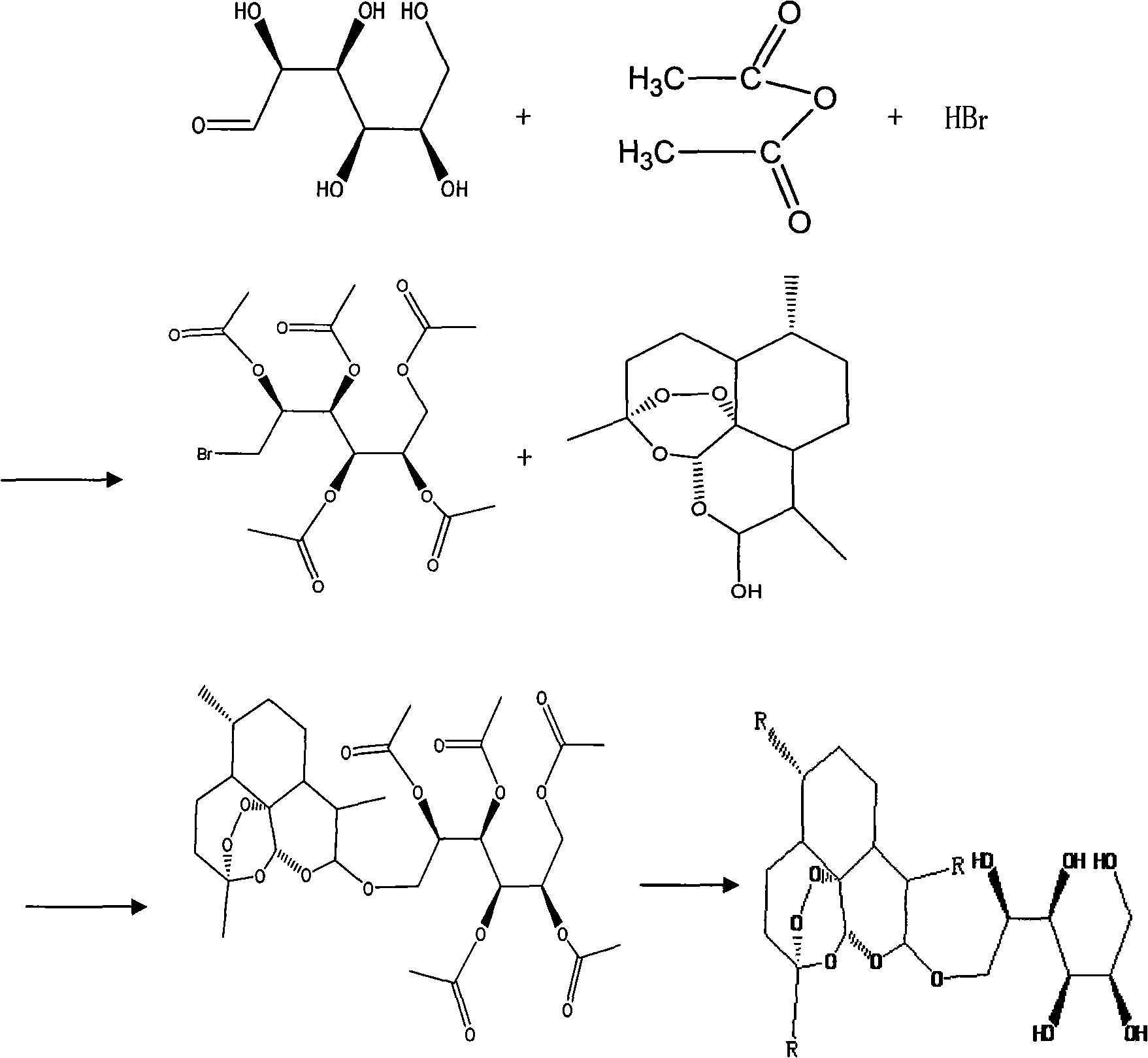
InactiveCN101293889AAddressing the thorny problem of poor treatment selectivityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilitySide effect
The invention relates to a water-soluble arteannuin derivative and a preparation method thereof. The method comprises the following steps of: (1) preparing dihydroarteannuin; (2) preparing tetra-acetyl-mannopyranosyl bromide; and (3) preparing the water-soluble arteannuin derivative. The water-soluble arteannuin derivative is proven to have good water solubility, is easy for preparing preparations and has good pharmaceutical prospect after structural identification and water solubility analysis. The inventive preparation method has the advantages of simple operation, mild reaction conditions, and no need of deprotection step. After initial cell experiments, the water-soluble arteannuin derivative is proven to have no toxic or side effects on normal cells.
Owner:CHONGQING UNIV
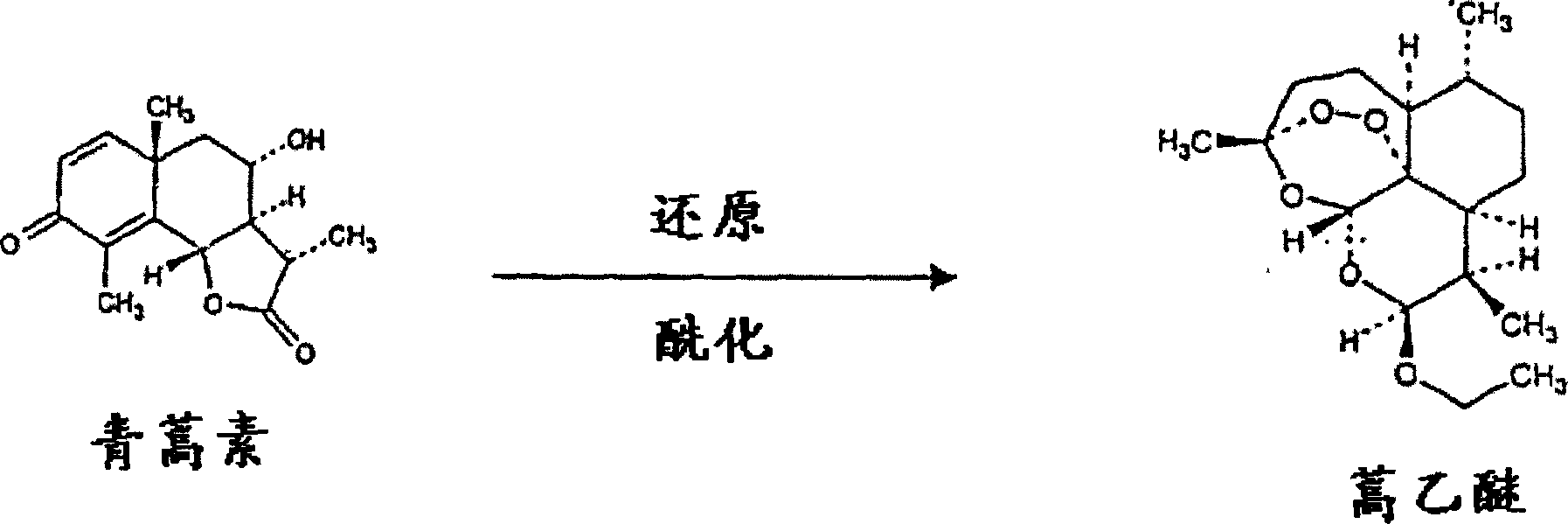
Single pot conversion of artemisinin into arteether
The present invention provides a method for the preparation of arteether from artemisinin in one pot in just about 4 hours comprising reduction of artemisinin into dihydroartemisinin by less quantity of sodium borohydride in ethanol at room temperature in the presence of a novel polyhydroxy catalyst, acylation of dihydroartemisinin in the presence of an acid catalyst, extraction of arteether from an aqueous reaction mixture using 1% ethyl acetate in n-hexane followed by workup and purification of the impure arteether to yield 80-86% (w / w) pure alpha, beta arteether.
Owner:COUNCIL OF SCI & IND RES
Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin
InactiveUS20050282804A1Easy to handleOrganic chemistryAntiparasitic agentsHalogenCompound (substance)
A novel one-stage process for preparing 10α-[4′-(S,S-dioxothiomorpholin-1′-yl)]-10-deoxo-10-dihydroartemisinin (1a) by reacting a compound of the formula (1b) in which X is a halogen atom with thiomorpholine dioxide at a temperature in the range of −30° C. to +20° C. is provided.
Owner:LANXESS DEUTDCHLAND GMBH
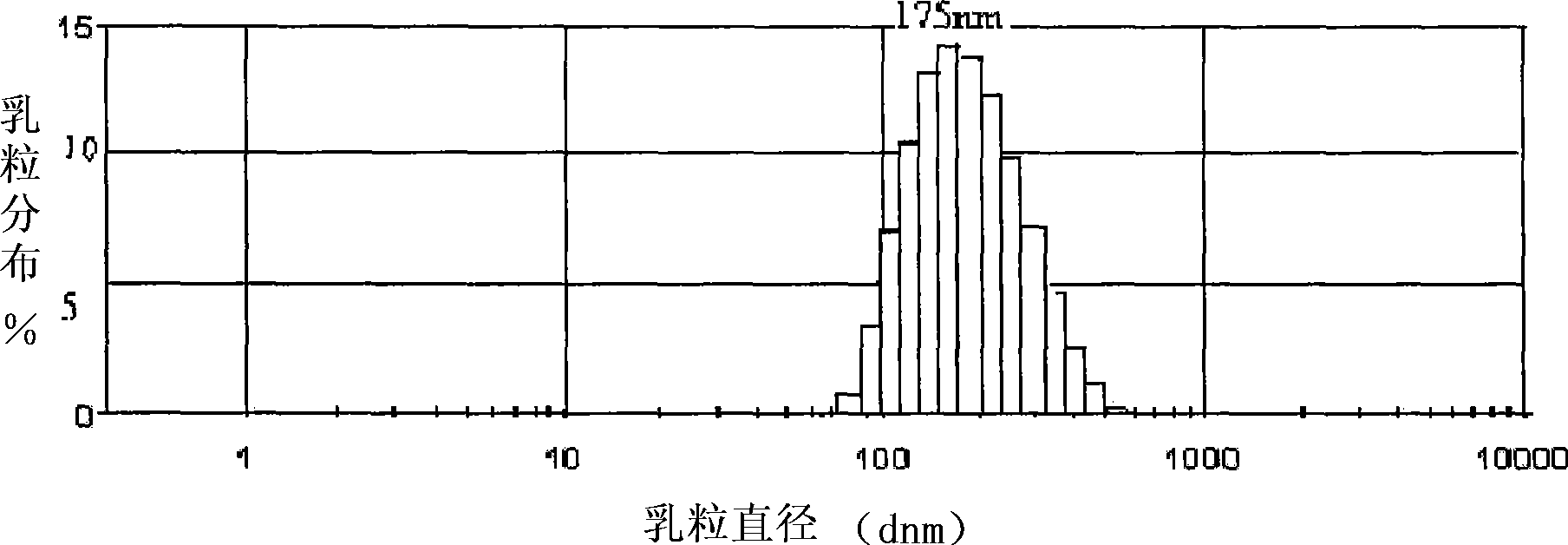
Dihydroartemisinin emulsion for injection, freeze-dried emulsion and preparation method thereof
InactiveCN101361711AImprove long-term stabilityAvoid layeringOrganic active ingredientsAntiparasitic agentsFreeze-dryingOil phase
The invention discloses an injection dihydroartemisinin emulsion, which is prepared by adjusting the pH value of the mixture consisting of the following raw materials with weight / volume percent based on the total volume of the emulsion: 0.05-0.3 percent of dihydroartemisinin, 5-30 percent of injection oil, 0.5-10 percent of emulsion, 0.1-3 percent of stabilizing agent, 0.5-5 percent of isoosmotic adjustment agent and the rest of injection water; the stabilizing agent consists of one or more of sodium taurocholate, sodium deoxycholate, oleic acid, sodium oleate and cholesterin and glycerol stearate. Freeze-dried emulsion can be made through freeze drying after adding freeze-dried protecting agent into the emulsion. The invention also discloses a method for preparing the emulsion as well as the freeze-dried emulsion. The injection dihydroartemisinin emulsion prepared by the method leads to the stable existence of dihydroartemisinin in the oil phase of the emulsion and has the advantages of good long term stability and curative effect, high bioavailability and accordance with the standards of intravenous injection; and the freeze-dried emulsion is convenient for carrying and storage and can further increase the stability of drugs.
Owner:ZHEJIANG UNIV
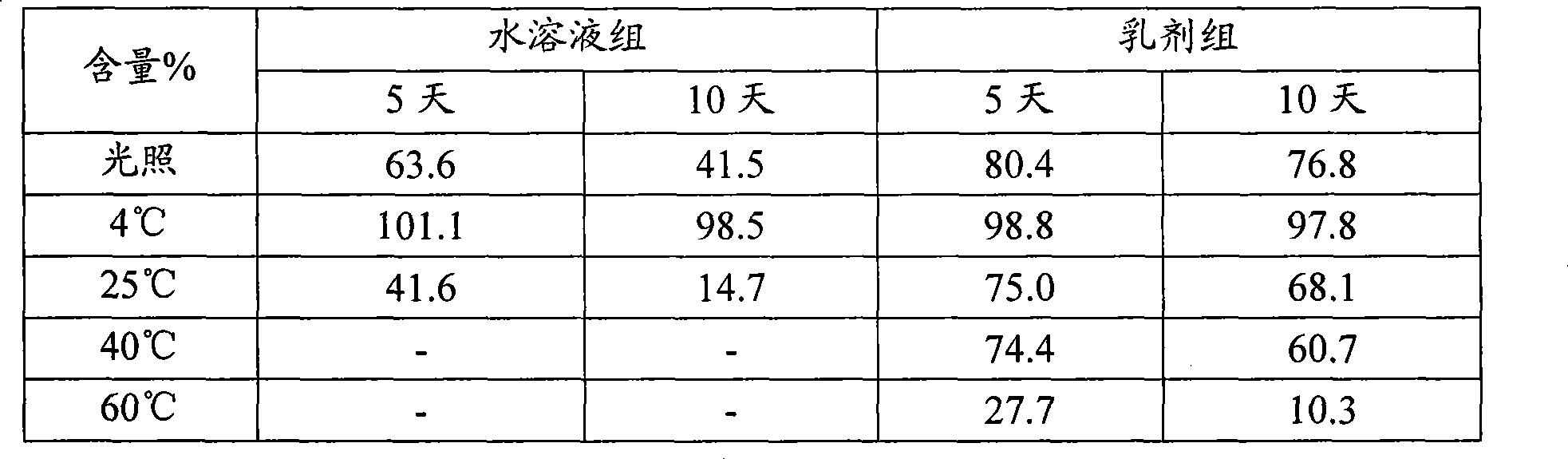
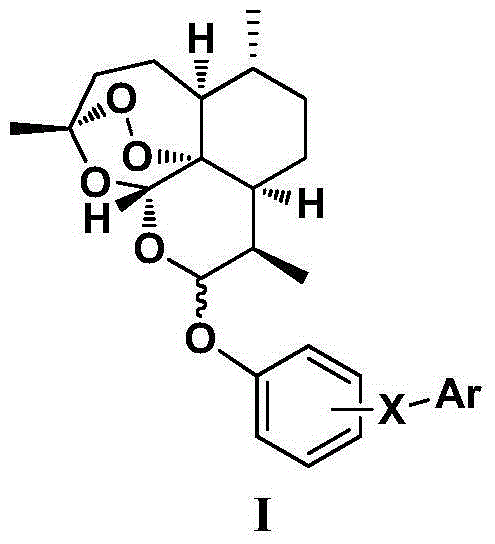
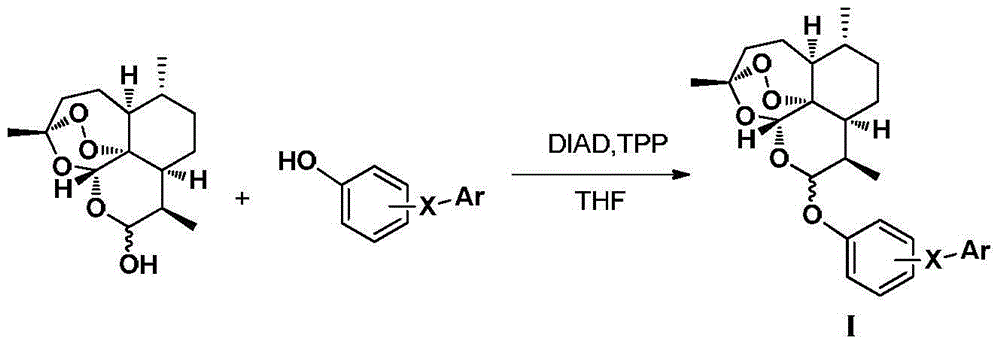
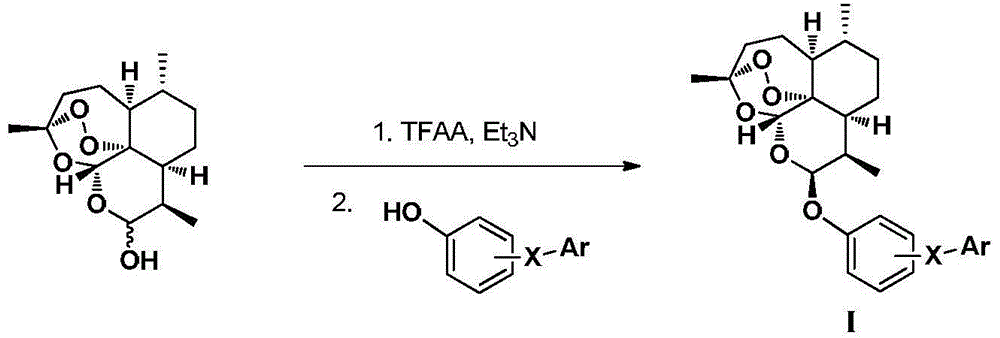
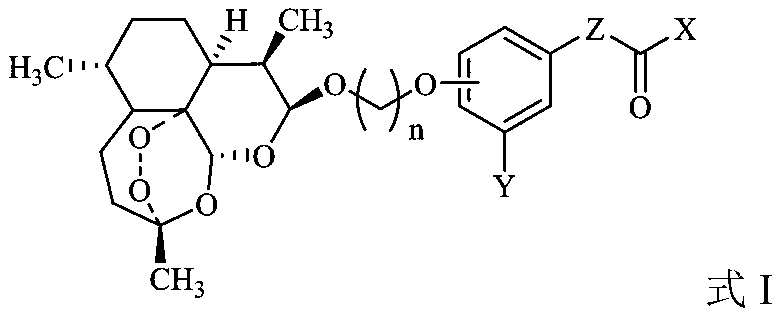
Dihydroartemisinin phenyl ether derivatives and applications thereof
InactiveCN104974171AAchieve separationStrong growth inhibitory effectOrganic active ingredientsOrganic chemistryCancer cellPhenyl Ethers
The invention relates to the technical field of medicine, and specifically relates to nitrogen-substituted dihydroartemisinin phenyl ether, optical isomers thereof and a preparation method thereof; pharmaceutical compositions with the derivatives as active components; and applications thereof in preparing medicines used for treating and / or preventing various cancers. The compound or pharmaceutically acceptable salts thereof have a structure as the following. The variables are as described in the claims and in the specifications. According to the invention, a pair of epimers with dihydroartemisinin C-10 site of R or S configuration can be prepared at a same time and with an equal amount, and separation can be realized. The prepared compounds have a significant effect in inhibiting cancer cell growth, and a selective killing effect against drug-resistant cells. The compounds have a potential of overcoming multidrug resistance.
Owner:SHENYANG PHARMA UNIVERSITY
Dihydroartemisinin emulsion for injection, freeze-dried emulsion and preparation method thereof
InactiveCN100579523CImprove long-term stabilityAvoid decomposition and inactivationOrganic active ingredientsAntiparasitic agentsFreeze-dryingOil phase
The invention discloses an injection dihydroartemisinin emulsion, which is prepared by adjusting the pH value of the mixture consisting of the following raw materials with weight / volume percent based on the total volume of the emulsion: 0.05-0.3 percent of dihydroartemisinin, 5-30 percent of injection oil, 0.5-10 percent of emulsion, 0.1-3 percent of stabilizing agent, 0.5-5 percent of isoosmotic adjustment agent and the rest of injection water; the stabilizing agent consists of one or more of sodium taurocholate, sodium deoxycholate, oleic acid, sodium oleate and cholesterin and glycerol stearate. Freeze-dried emulsion can be made through freeze drying after adding freeze-dried protecting agent into the emulsion. The invention also discloses a method for preparing the emulsion as well as the freeze-dried emulsion. The injection dihydroartemisinin emulsion prepared by the method leads to the stable existence of dihydroartemisinin in the oil phase of the emulsion and has the advantages of good long term stability and curative effect, high bioavailability and accordance with the standards of intravenous injection; and the freeze-dried emulsion is convenient for carrying and storage and can further increase the stability of drugs.
Owner:ZHEJIANG UNIV
Method for recycling mother solution generated in process of producing artemether
The invention provides a method for recycling mother solution generated in the process of producing artemether. In the conventional method for recycling alpha-artemether, the integral utilization rate of unit artemisinin and the total yield of beta-artemether are low. The method comprises the following steps of: evaporating artemether mother solution generated in the process of producing the artemether to remove methanol, performing concentration pretreatment, extracting by using a solvent, converting part of alpha-artemether and dihydroartemisinin into the beta-artemether in the presence of acid serving as a catalyst, neutralizing, washing, concentrating, and recrystallizing in a methanol-water system to generate the beta-artemether with high purity. The process is simple and high in recycling value and can be used for industrial production.
Owner:浙江来益生物技术有限公司
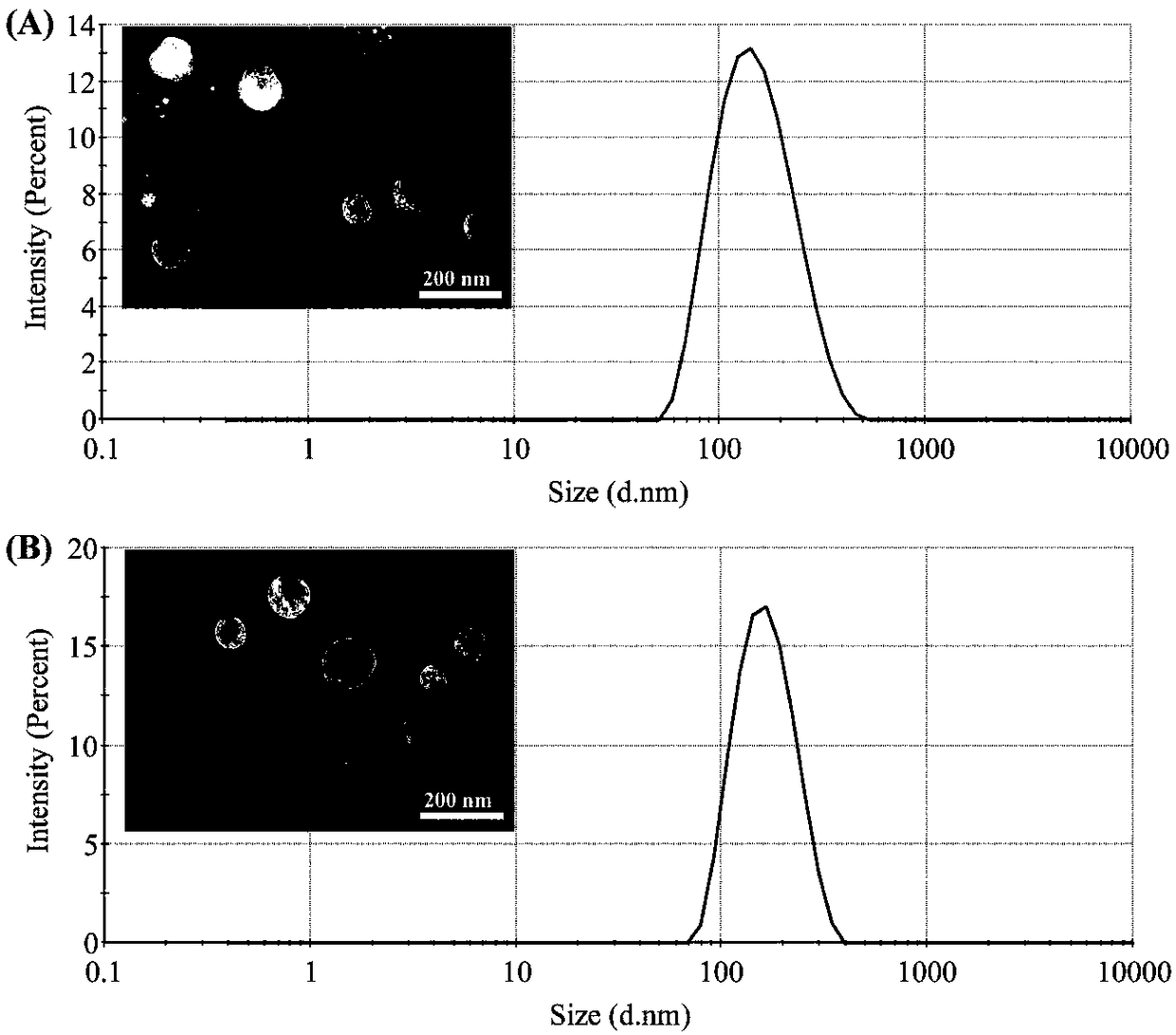
Dihydroartemisinin modified drug delivery carrier and application thereof in pharmaceutical science
ActiveCN108498485AImprove stabilityGood internal circulationOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolPharmaceutical formulation
The invention belongs to the field of new auxiliary materials and new dosage forms for pharmaceutical preparations and relates to design and application of a drug delivery system taking endogenous apolipoprotein E as a target spot, comprising design and synthesis of a carrier structure modifying a hydrophobic material by virtue of a polyethylene glycol (PEG) connecting arm by dihydroartemisinin (DHA). The used carrier material actively customizing apolipoprotein E takes DHA as a target head, PEG as a connecting arm and the hydrophobic material (such as PLGA) as an anchoring part. A nano delivery preparation prepared from the carrier material can encapsulate multiple anti-tumor drugs, and by virtue of interaction of the apolipoprotein combined with DHA on the surface of the nano delivery preparation and low density lipoprotein receptors (LDLr) highly expressed by tumor cells, multiple biological transmission barriers are overcome, and accumulation of nano particles at tumor parts as well as intake and anti-tumor activity in the tumor cells are effectively improved. The nano delivery preparation has good stability, high safety and excellent targeting capability, can be applied to intravenous injection and has a relatively great market application prospect.
Owner:SHENYANG PHARMA UNIVERSITY
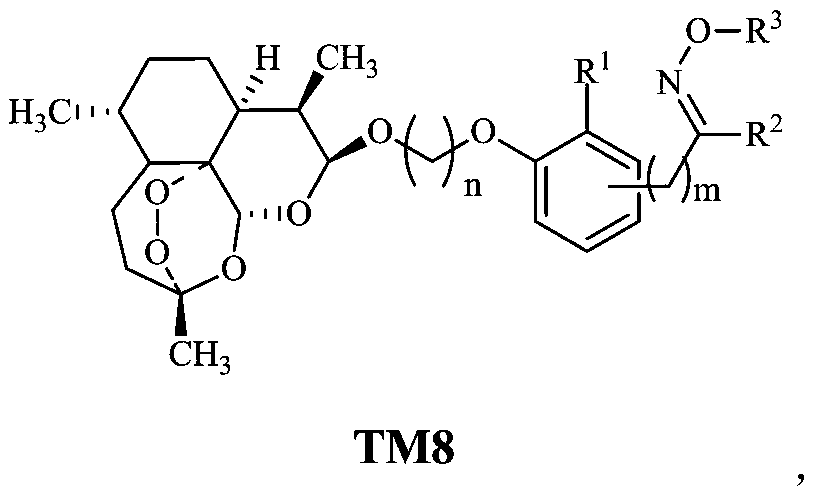
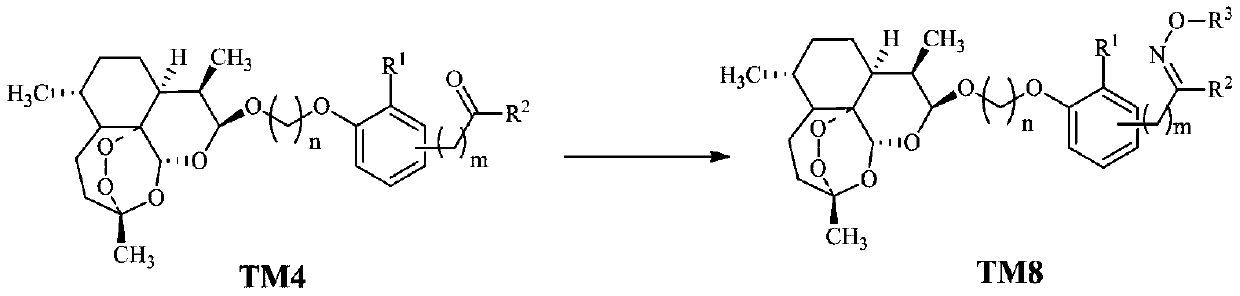
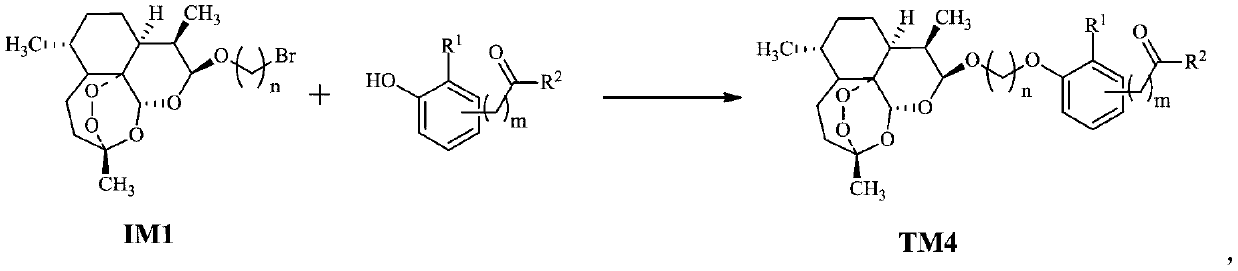
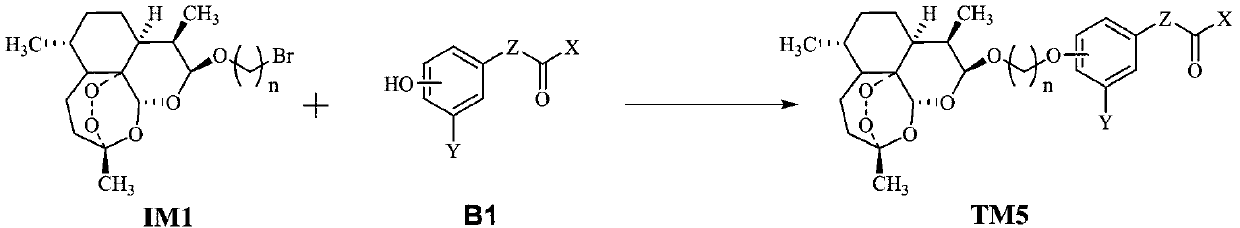
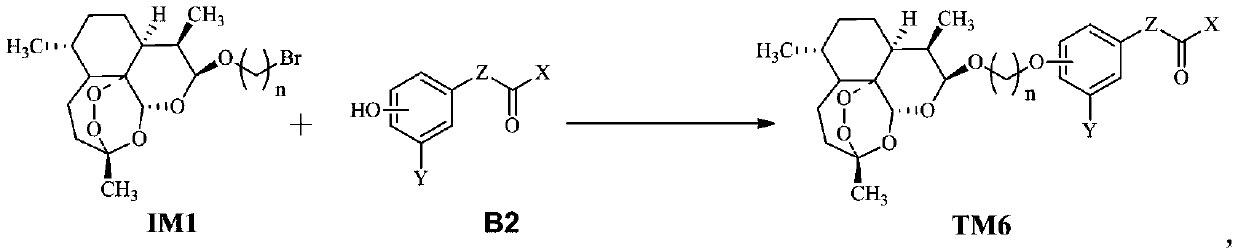
Dihydroartemisinin oxime-containing phenol derivatives as well as synthesis method and application thereof
ActiveCN110483545AAnti-diabeticWith hypolipidemicAntibacterial agentsOrganic active ingredientsSynthesis methodsInterleukin 17
The invention discloses dihydroartemisinin oxime-containing phenol derivatives as well as a synthesis method and application thereof, and belongs to the technical field of chemical medicines. The derivatives or racemates, stereoisomers, tautomers and pharmaceutically acceptable salts thereof have the following general formula shown in the specification. The invention also discloses the synthesis method of the derivatives, and the application of the derivatives in antituberculotic, antidiabetic, lipid-lowering and interleukin-17 inhibition drugs.
Owner:SOUTHWEST UNIVERSITY
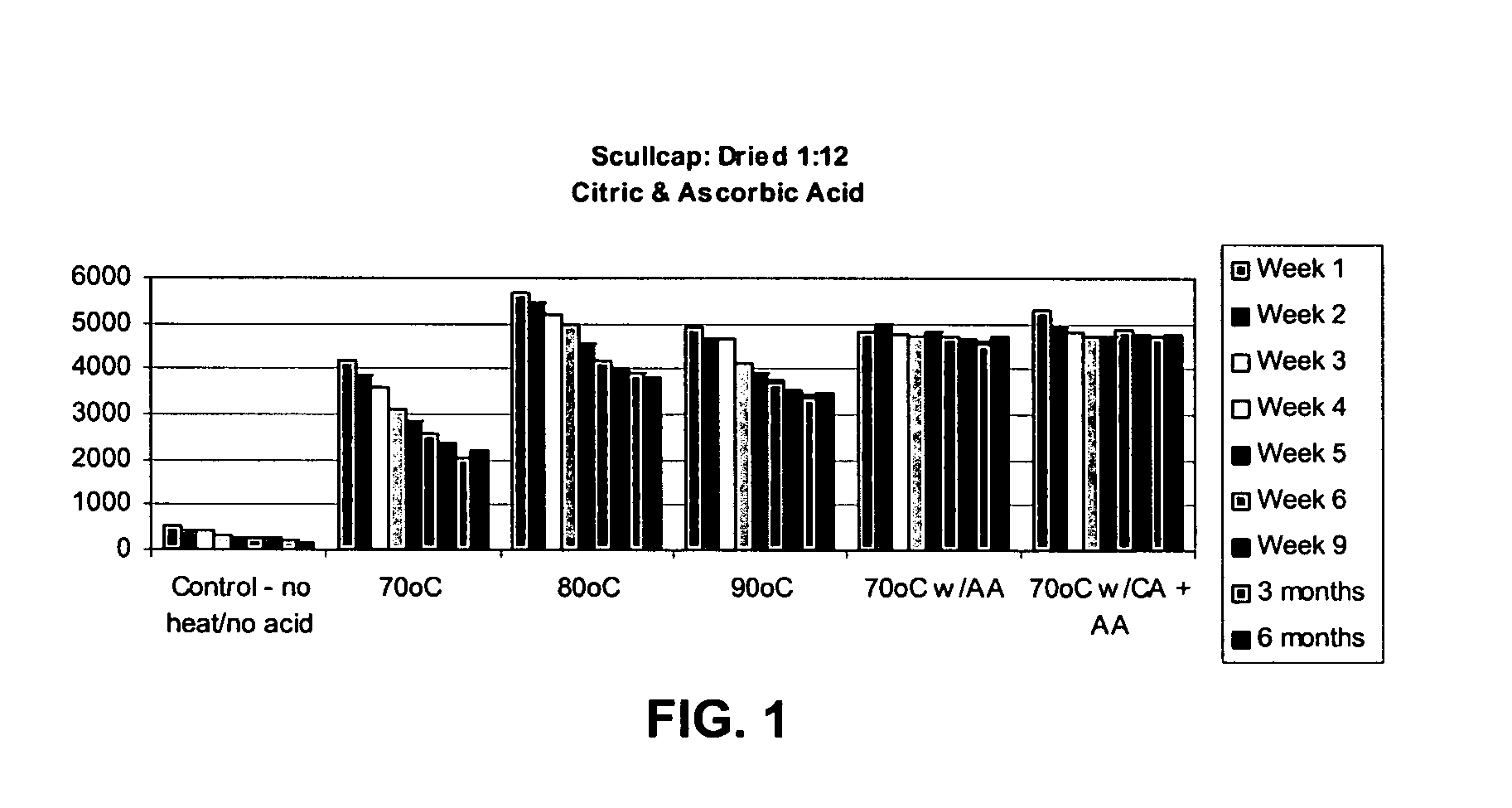
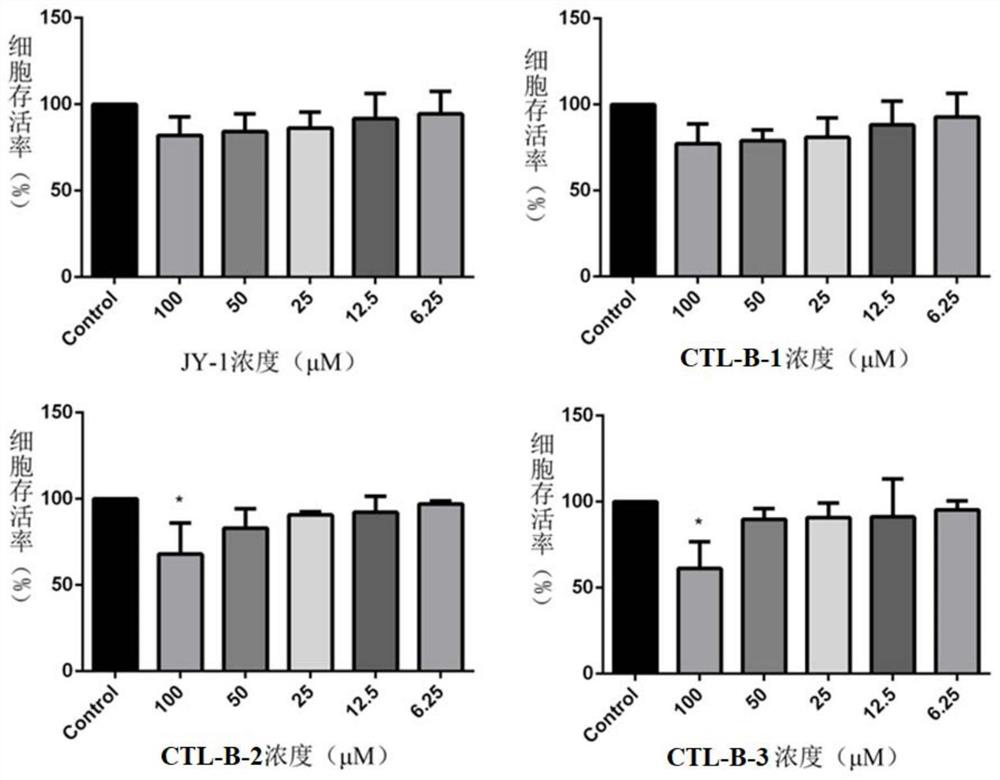
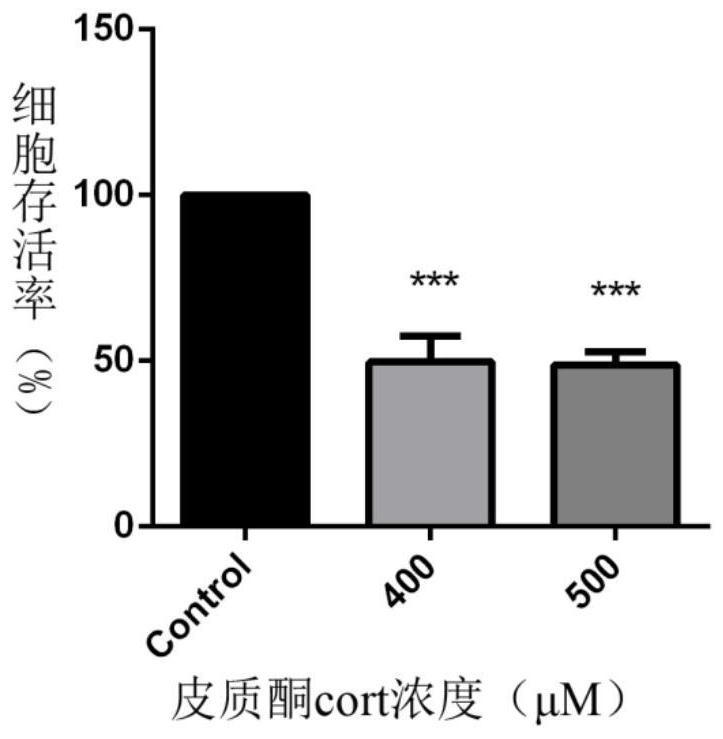
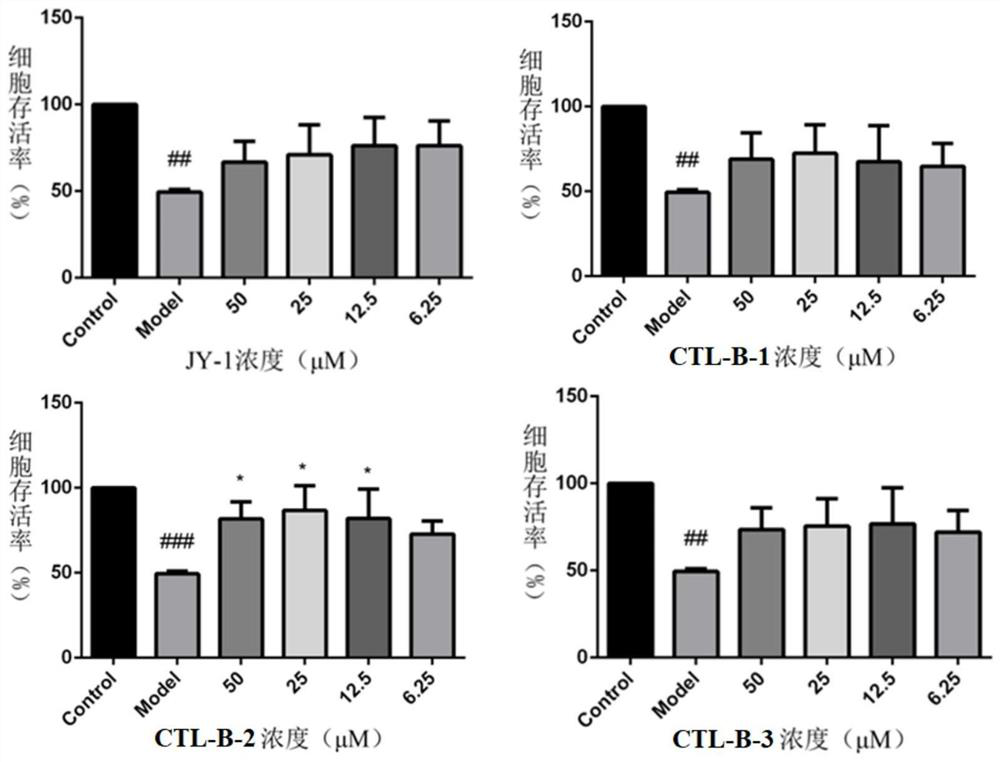
Extract of mad-dog skullcap
An extract of Scutellaria lateriflora L. having a content of flavonoids, calculated as the sum of baicalein, scutellarin, dihydrobaicalin, ikonnikoswide I, lateriflorin, baicalein, lateriflorein and wogonin, of at least 18% by weight. A method of obtaining such an extract includes combining dried Scutellaria lateriflora L. plant material with a solvent.
Owner:TOM'S OF MAINE
Dihydroartemisinin/neurotransmitter conjugate as well as synthesis method and application thereof
InactiveCN111747967ASimple preparation processEasy to industrializeOrganic active ingredientsNervous disorderSynthesis methodsDihydroartemisinin
The invention discloses a dihydroartemisinin / neurotransmitter conjugate as well as a synthesis method and application thereof, the structure of the dihydroartemisinin / neurotransmitter conjugate is shown as a formula I, wherein the symbol * refers to a beta-configuration, and R is a beta-aminopropionyl group, a gamma-aminobutyryl group or a 2-(1H-imidazole-4-yl)ethyl group. The compound disclosed by the invention is reported for the first time, has a treatment effect on neurodegenerative diseases, can be used for preparing medicines for treating neurodegenerative diseases, and particularly canbe used for preparing medicines for treating depression. Compared with other prior art, the preparation process of the compound is simple and is easy to industrialize.
Owner:广州药本君安医药科技股份有限公司
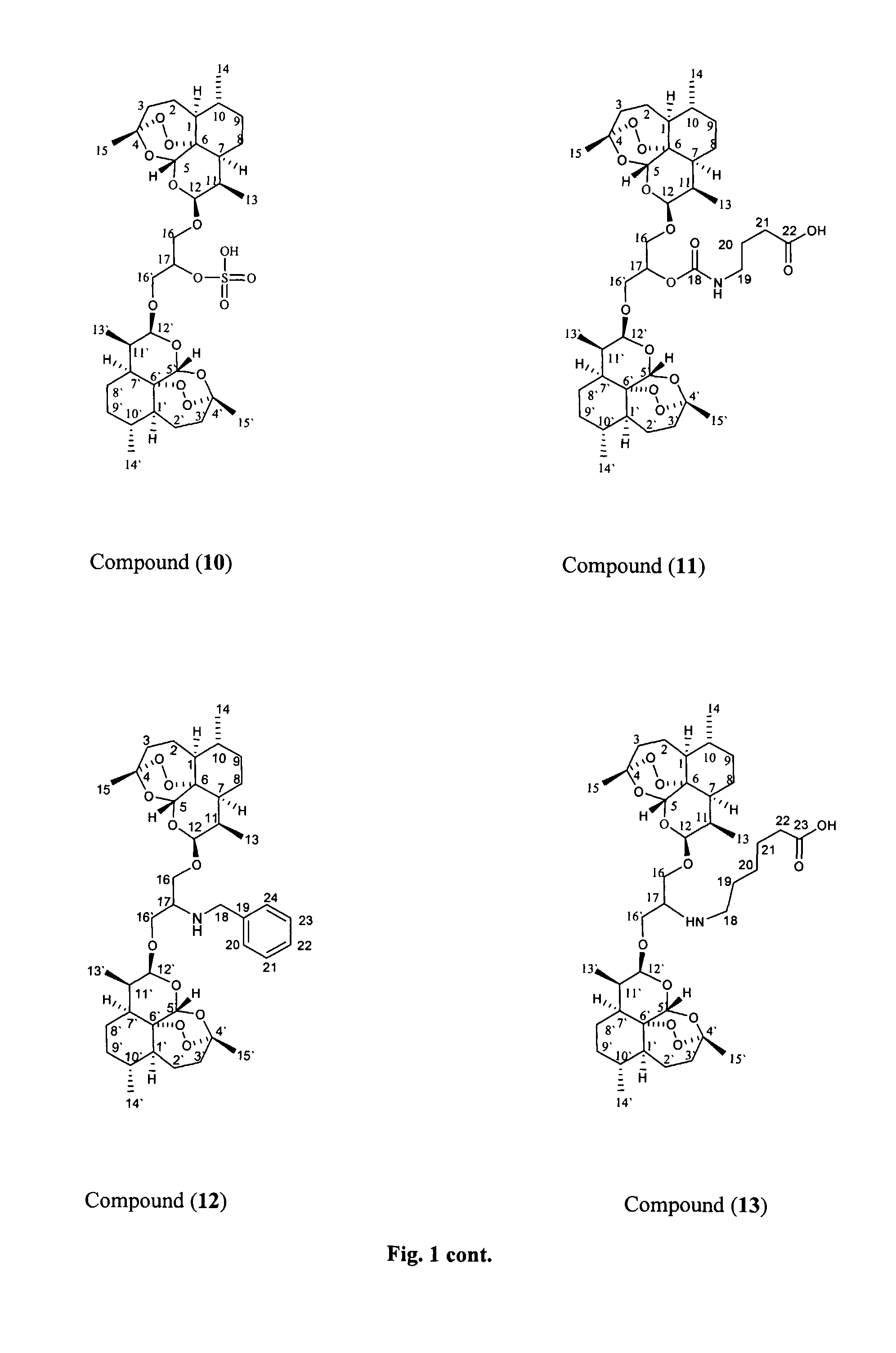
Anticancer and antiprotozoal dihydroartemisinene and dihydroartemisitene dimers with desirable chemical functionalities
This invention comprises compositions containing dihydroartemisinin- and dihydroartemisitene-dimers with activity as anticancer or anticancer metastasis agents and anti-protozal, including anti-malarial and anti-leishmanial properties. This invention also describes methods of preparation of these compositions and methods of use of such compositions for the treatment of cancer or prevention of cancer metastasis, and protozoal infections, including malaria, or leishmaniasis. The compounds of this invention represent a potential new class of anti-tumor or anti-metastasis agents, one that has shown promising activity against solid tumors.
Owner:ELSOHLY LAB
Dihydroartemisinin-containing carboxy phenol/ester phenol/amido phenol conjugate as well as synthesis method and application thereof
ActiveCN110590804AImprove biological activityHas anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsSynthesis methodsDihydroartemisinin
The invention discloses a dihydroartemisinin-containing carboxy phenol / ester phenol / amido phenol conjugate. The conjugate or a racemate, stereoisomer and tautomer thereof and a pharmaceutically acceptable salt of the conjugate have a structural general formula as shown in a formula I, wherein n is equal to 2 or 3, X is an alkoxyl, a hydroxyl, an amino group or -O(CH2)b-DHA, Y is -H or an alkoxyl,Z is an alkenyl and an alkyl or has no groups, b is equal to 2 or 3, and DHA represents for dihydroartemisinin. The invention further discloses a synthesis method of the dihydroartemisinin-containingcarboxy phenol / ester phenol / amido phenol conjugate and an application of the conjugate as a drug for resisting to tuberculosis and diabetes mellitus, reducing blood fat and inhibiting interleukin-17.
Owner:SOUTHWEST UNIVERSITY
Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin
A novel one-stage process for preparing 10α-[4′-(S,S-dioxothiomorpholin-1′-yl)]-10-deoxo-10-dihydroartemisinin (1a) by reacting a compound of the formula (1b)in which X is a halogen atom with thiomorpholine dioxide at a temperature in the range of −30° C. to +20° C. is provided.
Owner:LANXESS DEUTDCHLAND GMBH
Veterinary compound diminazene aceturate and artemisinin preparation and preparation technology thereof
ActiveCN102652753AQuality improvementFast absorptionOrganic active ingredientsPharmaceutical delivery mechanismSide effectGood manufacturing practice
The invention relates to a veterinary compound diminazene aceturate and artemisinin preparation, which is a powder injection comprising the following medicinal components in percentage by mass: 10 to 85 percent of diminazene aceturate, 5 to 60 percent of artemisinin or artemisinin extract, and 2 to 30 percent of beta-cyclodextrin. The preparation is prepared by the following steps of: 1) refining the diminazene aceturate; 2) preparing a water-soluble inclusion complex of the artemisinin or the artemisinin extract; and 3) proportioning, mixing, sterilizing and filling the preparation. The veterinary compound diminazene aceturate and artemisinin preparation has the advantages that the preparation is quick in absorption and high in bioavailability in subcutaneous or intramuscular injection; by combined utilization of the artemisinin and the diminazene aceturate which serve as active Chinese medicinal ingredients, a synergistic effect of Chinese medicaments can be achieved, and the toxic or side effect of the diminazene aceturate is reduced; the artemisinin, particularly the artemisinin extract is cheaper than dihydroartemisinin, artemether and artesunate; and the preparation is suitable to be produced in injection workshops of animal pharmaceutical factories with good manufacturing practice (GMP) conditions, and does not require to update the investment.
Owner:HUBEI WUDANG ANIMAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/DSA00000298513800011.png)
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/FSA00000298513900011.png)
![[(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof [(10S)-9,10-dihydroartemisinine-10-oxyl]benzaldehyde semicarbazones (sulfur) series substances as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/eb64c4a8-e8b1-40f0-8f34-a589ab94d906/BSA00000298514000011.png)
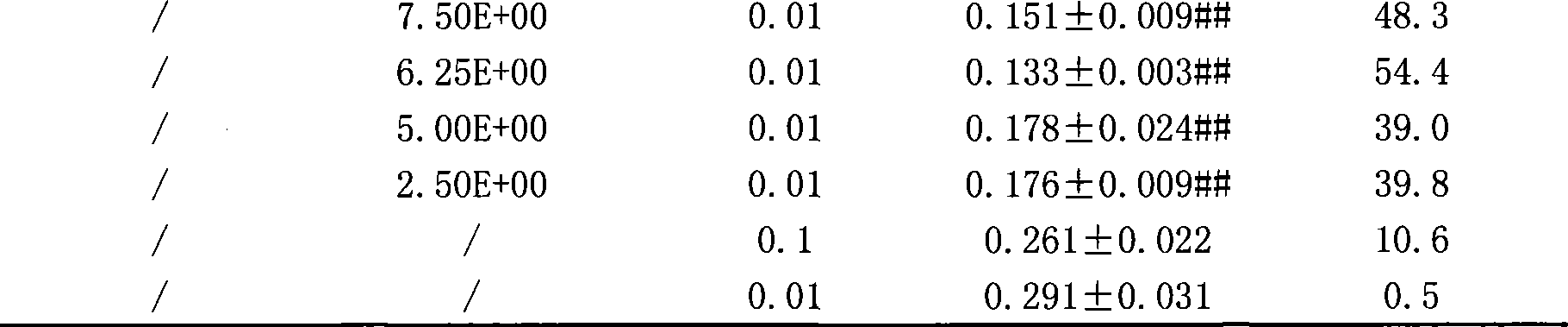
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/9a859e53-a507-48cf-b541-8a696d3f9d97/US20050282804A1-20051222-C00001.png)
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/9a859e53-a507-48cf-b541-8a696d3f9d97/US20050282804A1-20051222-C00002.png)
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/9a859e53-a507-48cf-b541-8a696d3f9d97/US20050282804A1-20051222-C00003.png)
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/a9b938c2-305a-4d41-a554-d04eec7dbb5f/US07241888-20070710-C00001.png)
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/a9b938c2-305a-4d41-a554-d04eec7dbb5f/US07241888-20070710-C00002.png)
![Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin Process for preparing 10alpha-[4'-(S,S-dioxothiomorpholin-1'-yl)]-10-deoxo-10-dihydroartemisinin](https://images-eureka.patsnap.com/patent_img/a9b938c2-305a-4d41-a554-d04eec7dbb5f/US07241888-20070710-C00003.png)