Dihydroartemisinin emulsion for injection, freeze-dried emulsion and preparation method thereof
A technology for dihydroartemisinin and injections, which is applied in the fields of emulsion delivery, anti-infective drugs, oil/fat/wax non-active ingredients, etc. It can solve problems such as unsuitable use, unsafe hidden dangers, and nephrotoxicity, and achieves Good long-term stability, avoid delamination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
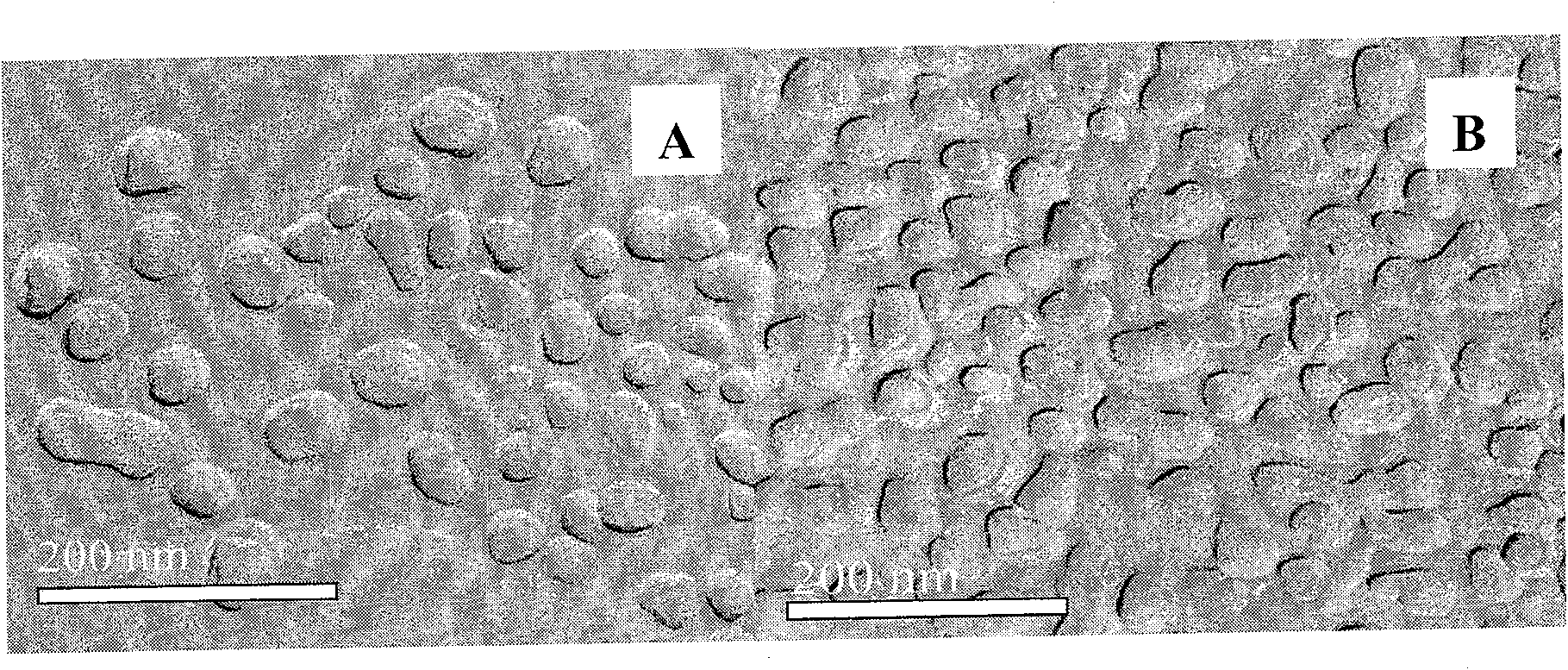
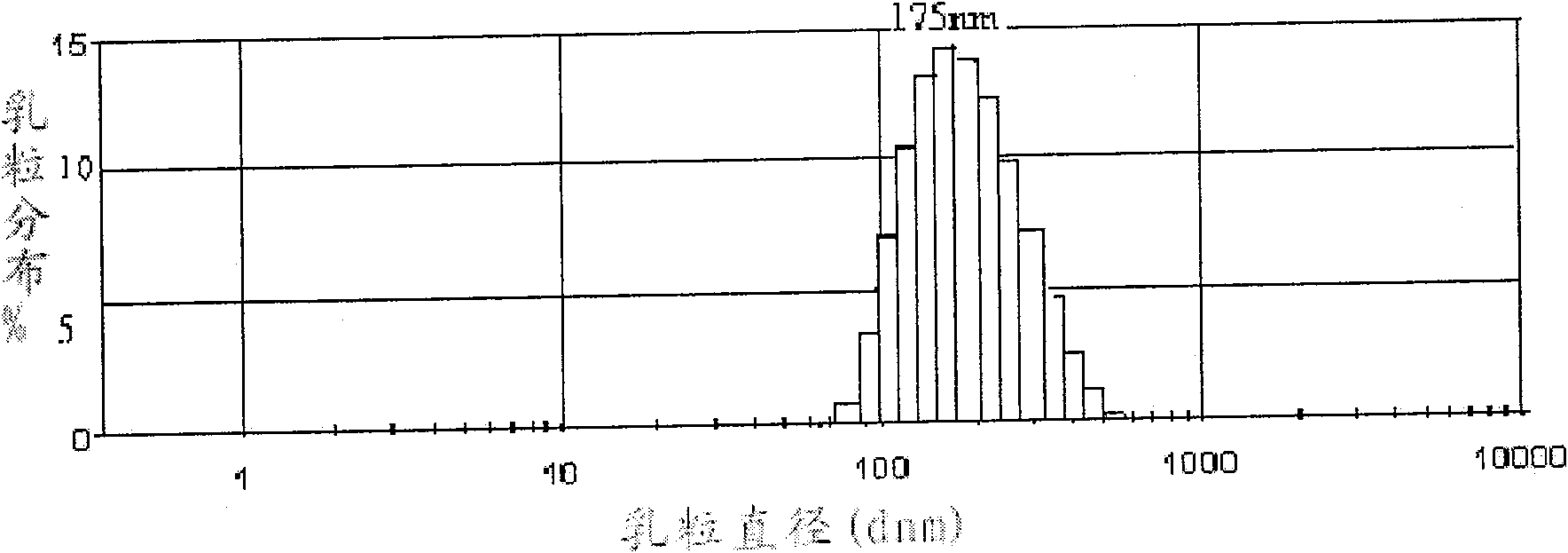
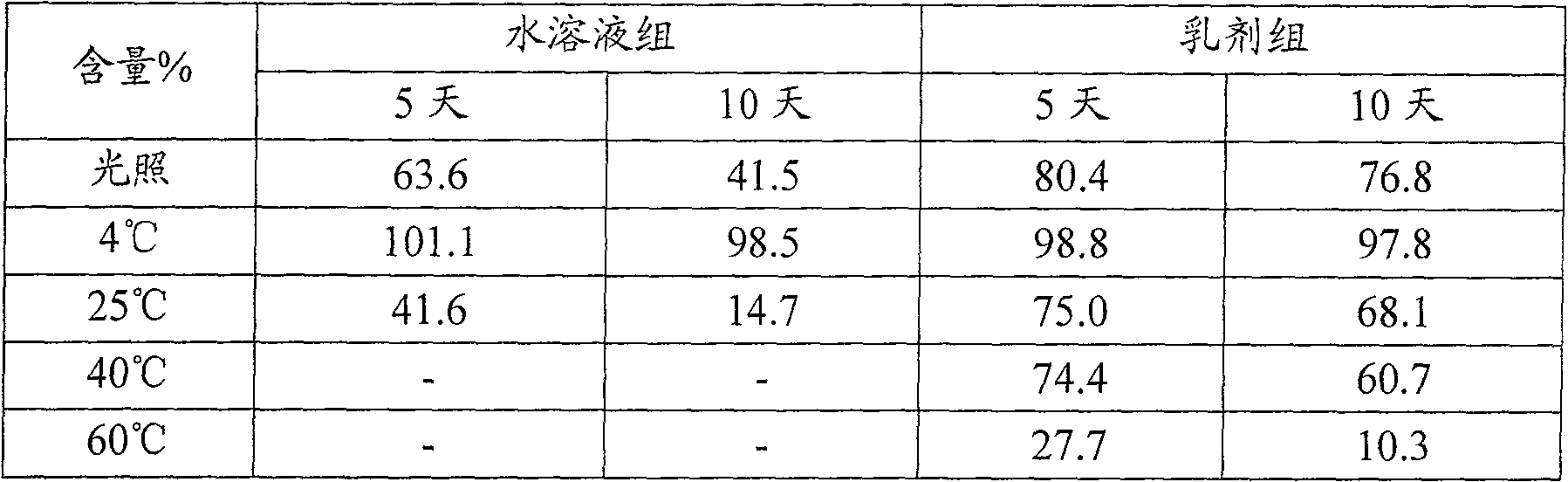
[0032]Take 0.05g of dihydroartemisinin, 0.25g of soybean lecithin, 0.10g of oleic acid, dissolve and mix evenly with an appropriate amount of ethanol at room temperature, evaporate to dryness under reduced pressure to remove ethanol, add 5g of soybean oil for injection, 0.05g of glycerol monostearate Mix the esters uniformly at 40°C to form oil phase A; take 80ml of water for injection, add 2.2g glycerin to it, stir and dissolve 0.25g F68 at room temperature to form water phase B; mix the above oil phase A with water phase B at 40°C , stirred by a high-speed homogenizer at the same temperature for 5 minutes to make colostrum, add water for injection to a final volume of 100ml, and adjust the pH value to 6-7.5; transfer the prepared colostrum into a micro-jet high-pressure homogenizer, and adjust it evenly Treat the pressure to 5000psi for 2 cycles, then adjust the homogenization pressure to 15000psi for 8 cycles to obtain the emulsion, pass the emulsion through a 0.22μm micropo...
Embodiment 2
[0034] Take 0.20g of dihydroartemisinin, 1.2g of soybean lecithin, 0.18g of oleic acid, dissolve and mix evenly with an appropriate amount of ethanol at room temperature, evaporate to dryness under reduced pressure to remove the ethanol, add 10g of soybean oil for injection, 0.10g of glycerol monostearate Mix the esters uniformly at 55°C to form oil phase A; take 80ml of water for injection, add 2.2g glycerin to it, and stir and dissolve 1.2g F68 at room temperature to form water phase B; mix the above oil phase A with water phase B at 55°C Mix and stir for 10 minutes with a high-speed homogenizer at the same temperature to make colostrum, add water for injection to a final volume of 100ml, and adjust the pH value to 6-7.5; transfer the prepared colostrum into a micro-jet high-pressure homogenizer, adjust Homogenize the pressure to 5000psi for 4 cycles, then adjust the homogenize pressure to 15000psi for 8 cycles to prepare the emulsion, pass the emulsion through a 0.22μm micro...
Embodiment 3
[0036] Take 0.25g of dihydroartemisinin, 1.8g of soybean lecithin, 0.8g of cholesterol, dissolve and mix evenly with an appropriate amount of ethanol at room temperature, evaporate to dryness under reduced pressure to remove ethanol, add 10g of soybean oil for injection, 0.10g of glyceryl monostearate in Mix well at 80°C to form oil phase A; take 80ml of water for injection, add 2.2g glycerin to it, and stir and dissolve 1.8g F68 at room temperature to form water phase B; mix the above oil phase A with water phase B at 80°C, Stir with a high-speed homogenizer at the same temperature for 5 minutes to make colostrum, add water for injection to a final volume of 100ml, and adjust the pH value to 6-7.5; transfer the prepared colostrum into a micro-jet high-pressure homogenizer to adjust the homogenization Treat the pressure to 5000psi for 4 cycles, and then adjust the homogenization pressure to 15000psi for 8 cycles to obtain the emulsion, pass the emulsion through a 0.22μm micropo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com