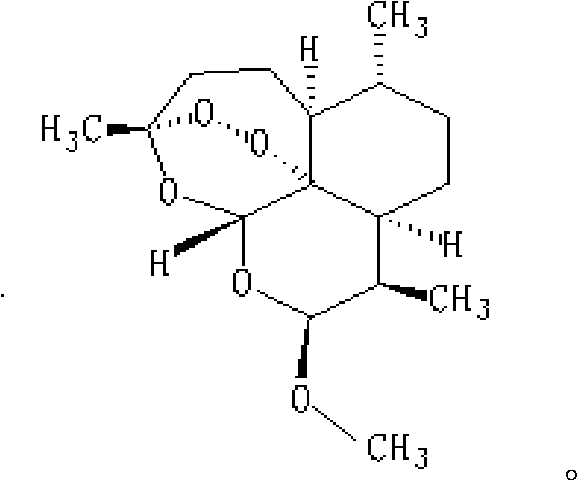
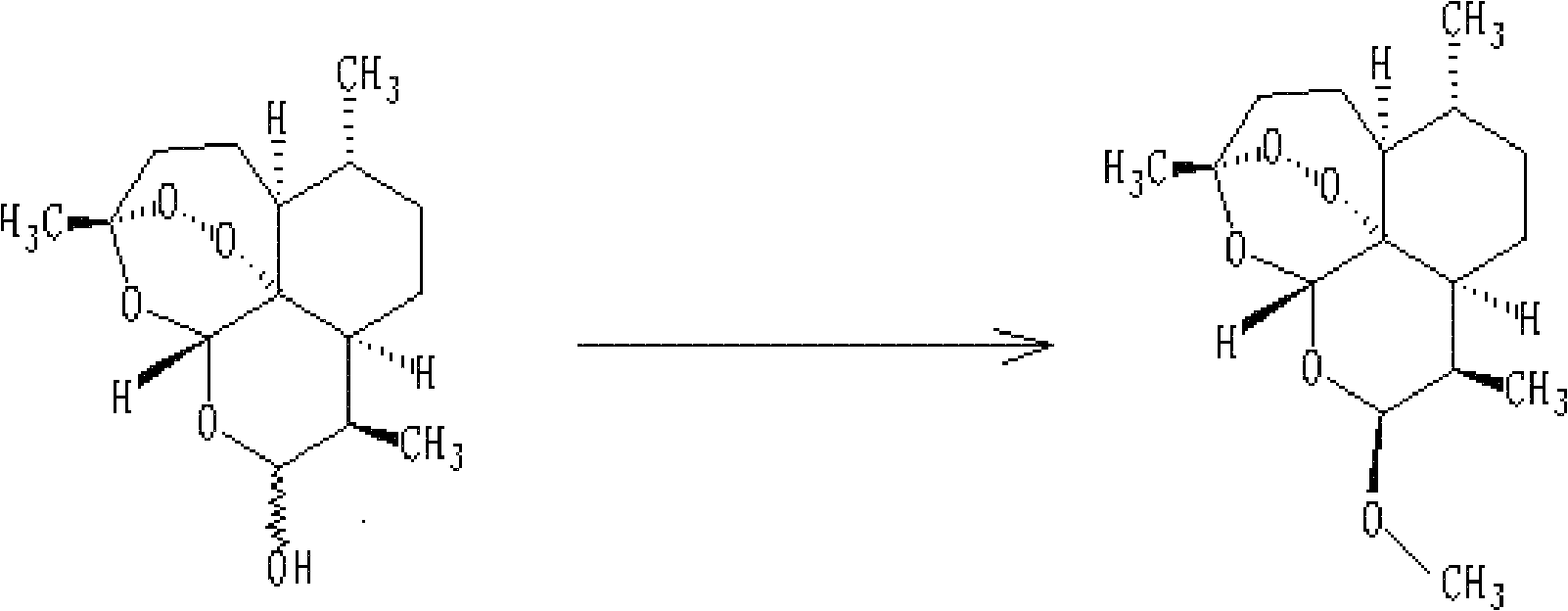
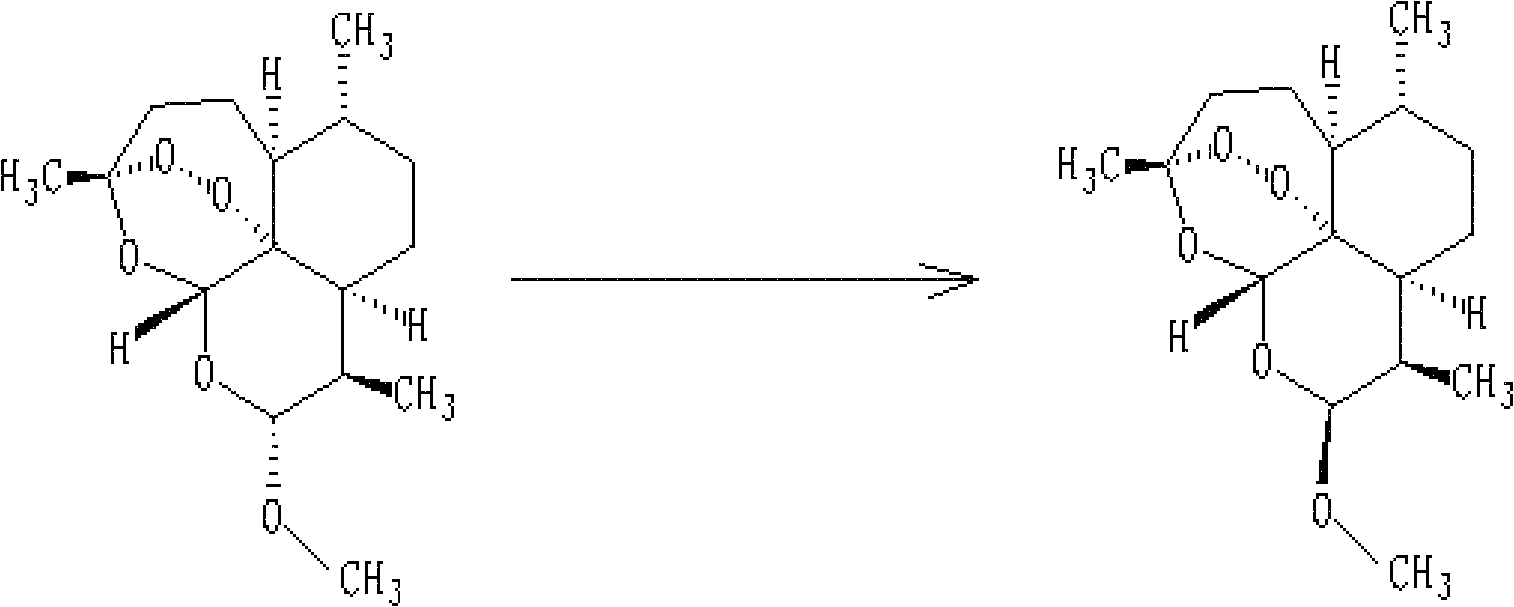
Method for recycling mother solution generated in process of producing artemether
A production process, artemether technology, applied in the field of mother liquor recycling, can solve the problems of low yield, no practical value, and many impurities, achieve huge social value and economic value, and improve the overall utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Extraction of recyclable substances in artemether mother liquor: 3450ml of mother liquor produced by converting dihydroartemisinin into artemether and washing by rejection filtration was concentrated under reduced pressure at a temperature lower than 40°C in vacuum, and evaporated for about 7 hours. After removing most of the methanol (about 1500ml~2000ml), put ~1350ml of n-heptane into the remaining (1450ml~1950ml) concentrated solution, stir for half an hour, then let stand to separate the water layer (1450ml~1950ml), keep Organic layer 1450ml carries out next step reaction.
[0034]Conversion reaction of recyclable substances: 450ml of n-heptane layer solution, 80ml of methanol and 2.5ml of concentrated HCl were put into a 1000ml four-necked flask, and the reaction was started at a temperature of 30°C. Sampling liquid phase analysis every hour, the content of α-artemether and β-artemether will not change after 8h~12h (α-artemether is 15%~20%, β-artemether is 50%~55% ...
Embodiment 2
[0037] The conversion reaction of recyclable substances: 450ml of n-heptane layer solution, 80ml of methanol and 2.5ml of perchloric acid were put into a 1000ml four-neck flask, and the reaction was started at a temperature of 30°C. Sampling liquid phase analysis every hour, the content of α-artemether and β-artemether will not change after 6h~8h (α-artemether is 12%~16%, β-artemether is 57%~62% %), began to reduce the reaction temperature to 12 ° C ~ 15 ° C.
[0038] Extraction of β-artemether: Add 200ml of about 5% sodium bicarbonate solution to the reaction solution, adjust the pH to neutral, stir for 30min and separate the layers, keep the n-heptane layer for concentration under reduced pressure. Concentrate under vacuum at a temperature of 40°C until it becomes viscous. If it cannot be viscous, it can be entrained with anhydrous methanol until there is basically no organic solvent. For the last concentration, methanol can be used to completely dissolve the viscous matter...
Embodiment 3
[0040] Conversion reaction of recyclable substances: put 450ml of n-heptane layer solution, 80ml of methanol and 2.5ml of concentrated sulfuric acid into a 1000ml four-neck flask, and start the reaction at a temperature of 30°C. Sampling liquid phase analysis every hour, the content of α-artemether and β-artemether will not change after 4h~7h (α-artemether is 12%~18%, β-artemether is 57%~66%) %), began to reduce the reaction temperature to 12 ° C ~ 15 ° C.
[0041] Extraction of β-artemether: Add 200ml of about 5% sodium bicarbonate solution to the reaction solution, adjust the pH to neutral, stir for 30min and separate the layers, keep the n-heptane layer for concentration under reduced pressure. Concentrate under vacuum at a temperature of 40°C until it becomes viscous. If it cannot be viscous, it can be entrained with anhydrous methanol until there is basically no organic solvent. For the last concentration, methanol can be used to completely dissolve the viscous matter an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com