Veterinary compound diminazene aceturate and artemisinin preparation and preparation technology thereof
A compound triazamidine and preparation process technology, which can be applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. Toxic and other problems, to achieve the effects of fast absorption, high bioavailability, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
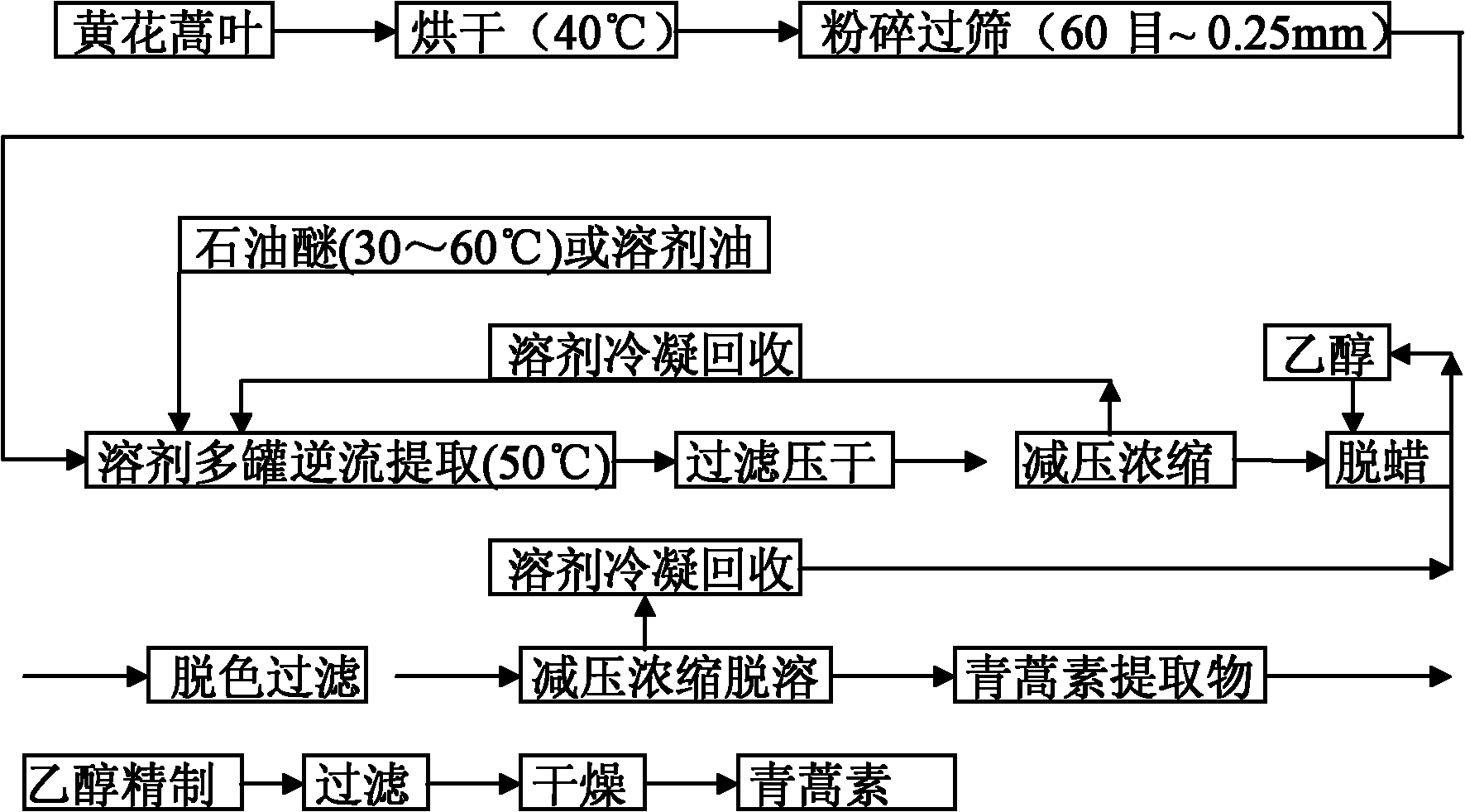
[0045] Take the leaves of Artemisia annua that produce artemisinin content of more than 0.2% in the Yunxi area of Hubei Province, put them in a drying box and dry them at 40°C, and TLC The law distinguishes its authenticity. Crush and pass through a 60-mesh-0.25mm sieve. According to the literature (Li Chunli et al., [J], Journal of Chongqing Medical University, 2007, 32 (4), 413), the content of artemisinin was determined by ultraviolet spectrophotometry, and the content of artemisinin was required to be more than 0.2%.
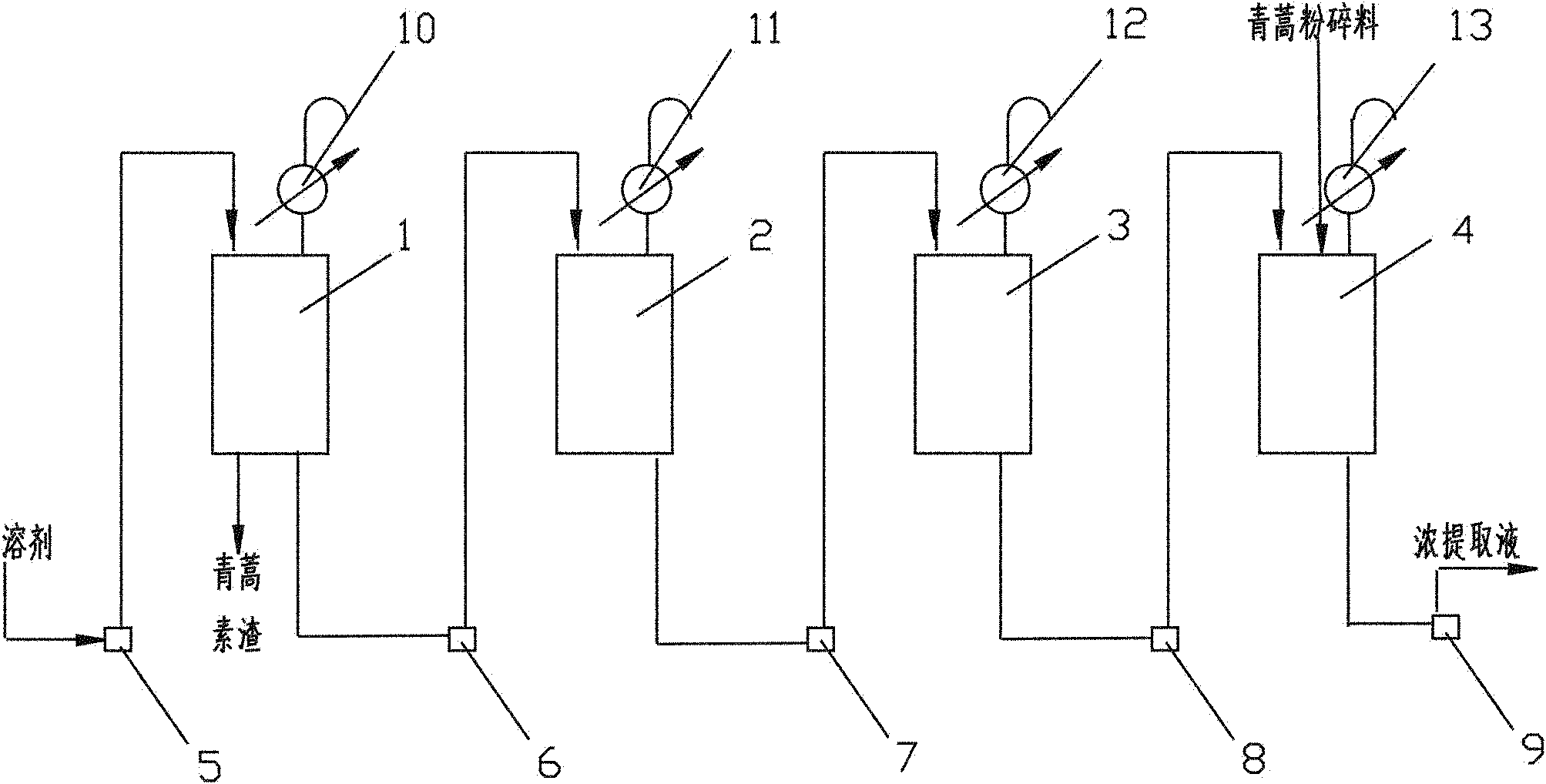
[0046] Weigh about 10Kg of powder into the extraction tank group, such as figure 2 shown, press figure 1 Process and process parameters countercurrent extraction (extraction tank adopts extraction temperature 50°C; extraction time 60min; stirring speed 200-400 rpm; extraction solvent is 50°C petroleum ether; the solid-liquid ratio of the leaves and flower buds of Artemisia annua to the extraction solvent is 1 :9). The solvent rich in active ingredient...
Embodiment 2
[0051] Example 2 follows the same operating method and process conditions as in Example 1, except that artemisinin is replaced by artemisinin extract.
Embodiment 3
[0052] Example 3 is the same operating method and process conditions as in Example 1, except that it is composed of the following composition and mass percentage: triazamidine 15%; artemisinin or artemisinin extract 55%; β-cyclodextrin 30% (both are mass percentages), prepared, mixed, potted and sterilized to make compound triazamidine and artemisinin injection powders with specifications of 0.5-4g / bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com