Aglycosyl anti- CD154 (CD 40 ligand) antibodies and uses thereof
One-CD154, antibody technology, applied in the field of non-glycosyl anti-CD154 antibody or its antibody derivatives, can solve problems such as inappropriate platelet activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Generation and evaluation of the aglycosyl hu5c8 antibody
[0157] Production and expression of aglycosyl hu5c8 mAb
[0158] To reduce the effector function of hu5c8 mAb, the aglycosylated form was modified by changing the heavy chain C H2 The classical N-linked Asn site in the domain was created for the Gln residue.
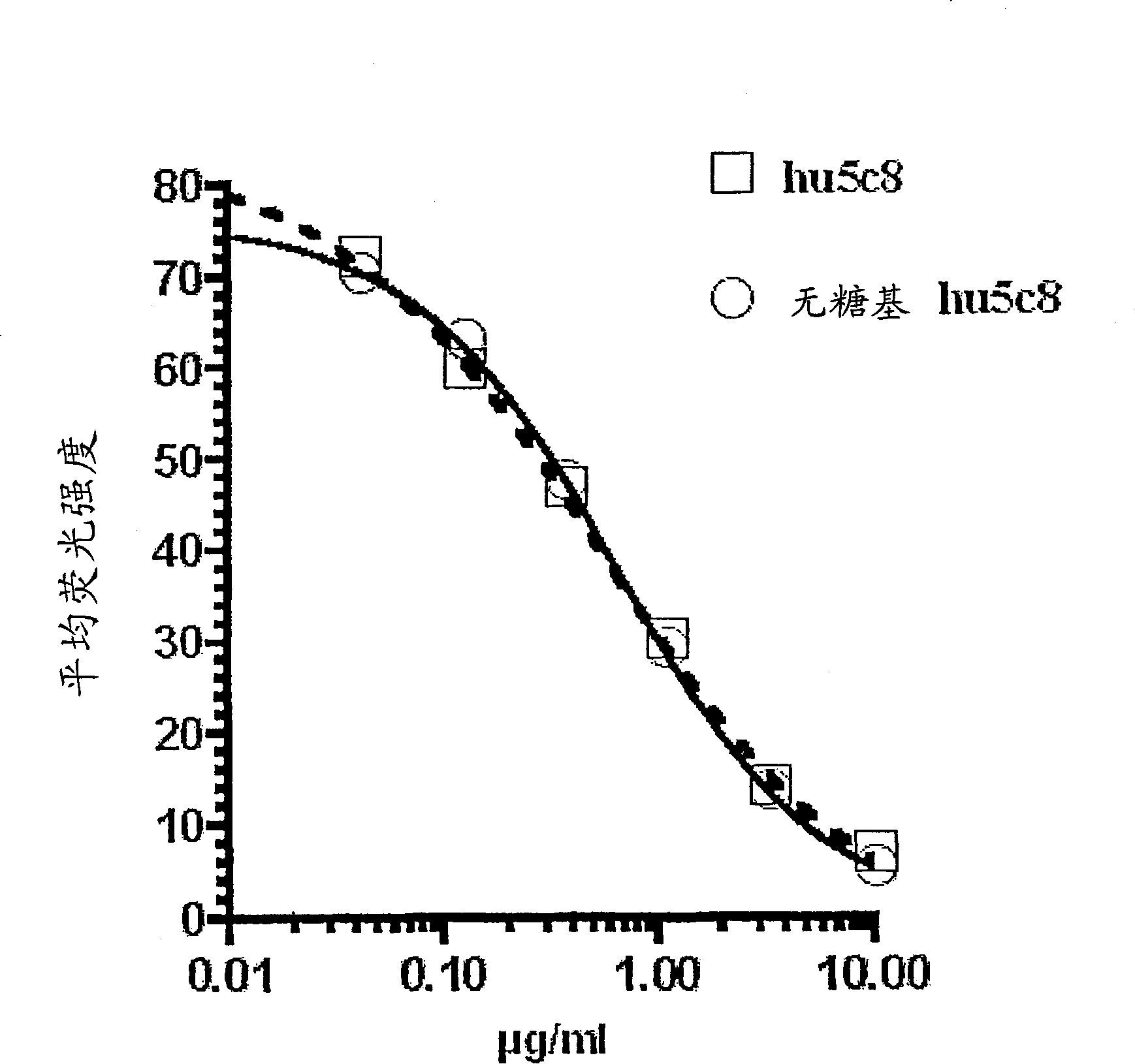
[0159] Competition binding assays confirmed that the ability of the aglycosylated hu5c8 mAb to bind cell surface CD154 was not altered compared to the glycosylated hu5c8 mAb ( figure 1 ).
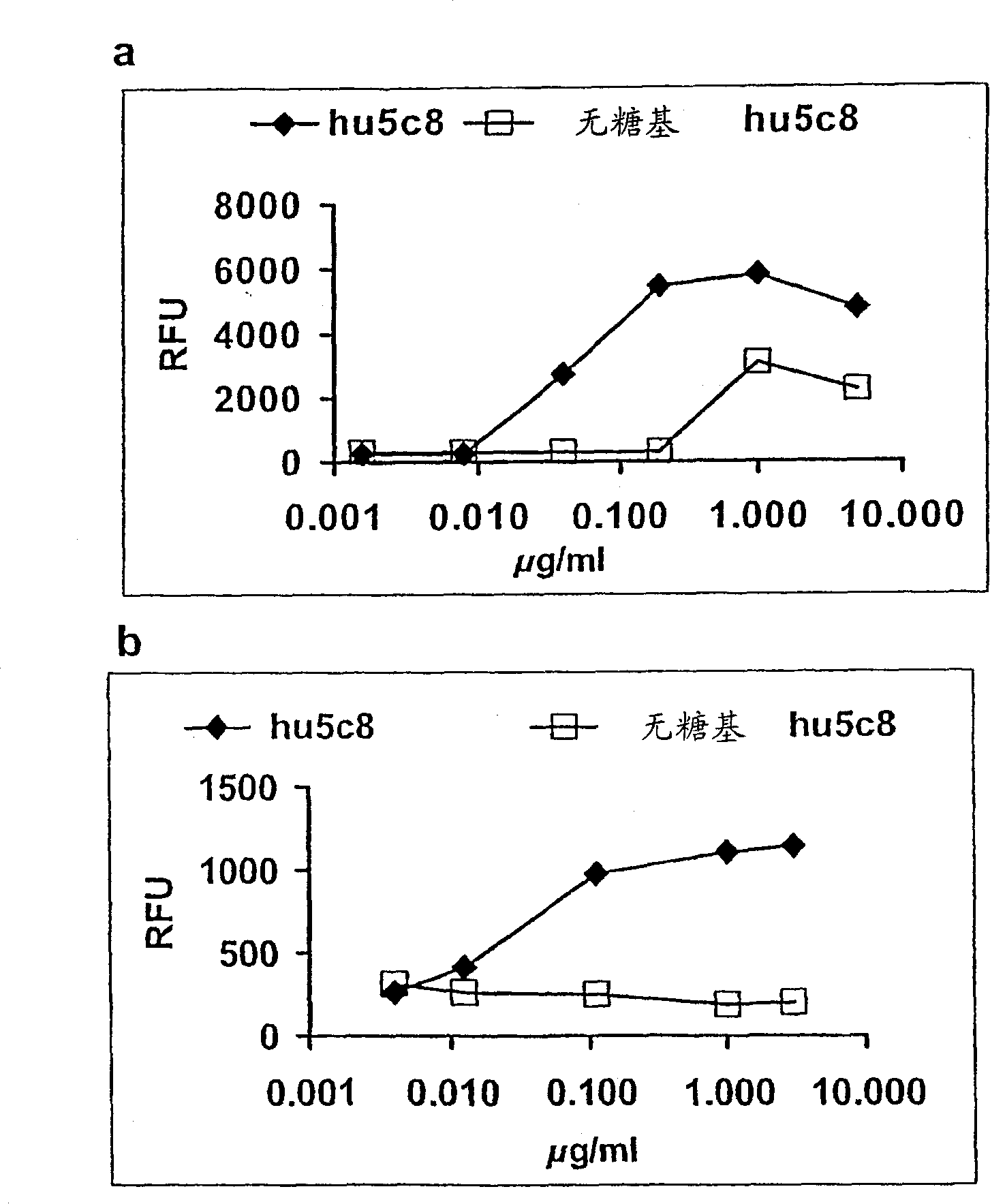
[0160] Effector function was assayed in vitro using a bridging assay format. Compared with glycosylated hu5c8mAb, the relative binding of aglycosylated hu5c8mAb to FcγRI was weakened 25-fold ( figure 2 A). Residual binding of the aglycosylated hu5c8 mAb to FcγRIII could not be demonstrated at concentrations up to 5 mg / ml, whereas the normally glycosylated hu5c8 mAb showed an EC50 of 50 ng / ml in the same assay format ( figure 2 B).
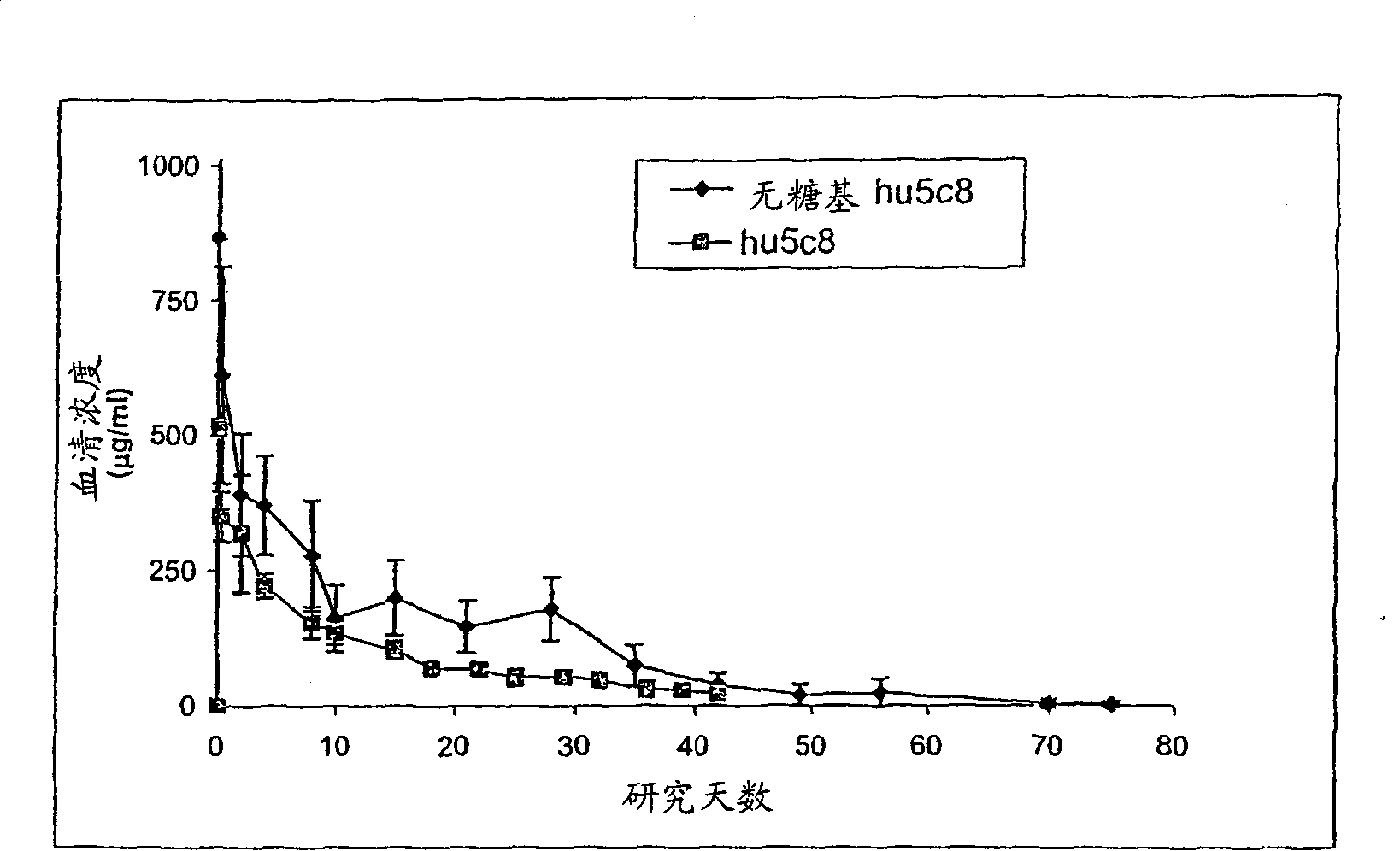
[0161] Pharmacok...
Embodiment 2
[0174] Example 2: Aglycosyl hu5c8 antibody inhibits initial and subsequent humoral responses
[0175] Suppression of the initial humoral immune response to tetanus toxoid (TT) antigen in macaques
[0176] The ability of aglycosylated hu5c8 mAb and glycosylated hu5c8 mAb (prepared according to Example 1 ) to inhibit the initial antibody response to TT, each at a single dose of 20 mg / kg, was assessed in separate experiments. Administration of aglycosylated hu5c8 mAb or glycosylated hu5c8 mAb resulted in an overall primary immune response compared to saline-treated controls (E AUC ) were reduced by 70% and 77%, respectively. Figure 4 Graph showing TT antibody titers throughout 42 days, showing that the aglycosylated hu5c8 mAb inhibited the initial humoral response to a similar extent as the glycosylated hu5c8 mAb, but with reduced FcγR binding.
[0177] The immunogenicity of humanized mAbs is another measure of their effectiveness in this non-human primate model. Three of fou...
Embodiment 3
[0199] Example 3: Aglycosyl muMR1 antibody inhibits lupus nephritis
[0200] Systemic lupus erythematosus ("SLE") is a spontaneously occurring autoimmune disease, predominantly in women, characterized by the production of multiple pathological antinuclear autoantibodies. In lupus nephritis, renal injury is primarily mediated by a combination of cellular and humoral immune mechanisms, including the formation of immune complexes that deposit in glomeruli and activate the complement cascade, which leads to glomerulonephritis. It was previously determined that antinuclear antibody production in both human and mouse SLE is driven by cognate interactions between selected populations of autoimmune Th and B cells. [Kalled et al., 1998].
[0201] Previous studies demonstrated that chronic treatment of hamster MR1 (haMR1) with an anti-CD154 mAb in (SWR x NZB) F1(SNF1) mice with established lupus nephritis prolongs survival and reduces severe incidence of nephritis.
[0202] In this e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com