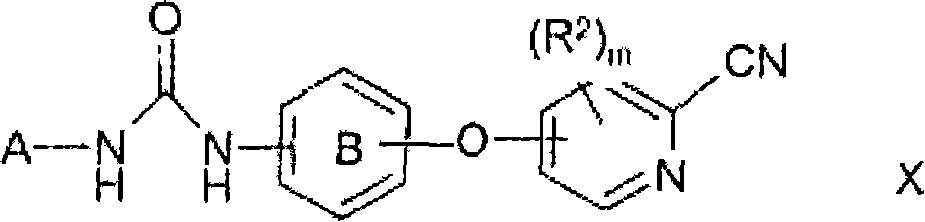
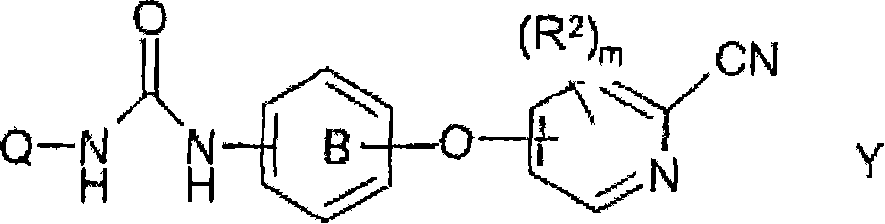
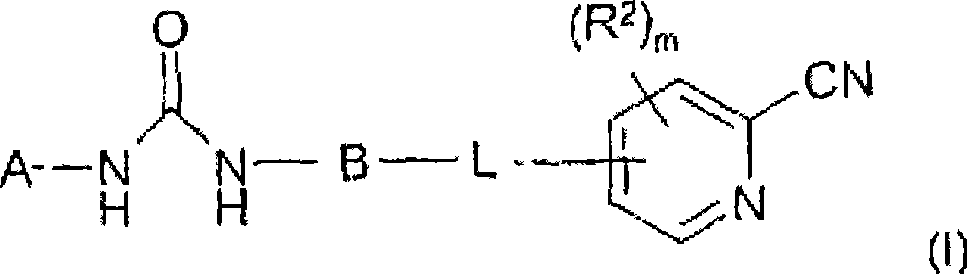Novel cyanopyridine derivatives useful in the treatment of cancer and other disorders
A cyano- and pyridyl-based technology, which is applied in the field of treatment of hyperproliferative and angiogenic diseases, and can solve problems such as hyperproliferative blood vessels and blindness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0421] Unless otherwise stated, all reaction conditions, methods, abbreviations and reagents are as follows. All temperatures are in degrees Celsius (° C.) and all parts and percentages are by weight. Technical grade reagents and solvents were used without further purification.
[0422] Abbreviations used in this manual
[0423] DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
[0424] DMF N,N-Dimethylformamide
[0425] DCM dichloromethane
[0426] DCE 1,2-Dichloroethane
[0427] DMSO Dimethyl Sulfoxide
[0428] HPLC high pressure liquid chromatography
[0429] MPLC Medium Pressure Liquid Chromatography
[0430] LC-MS Liquid Chromatography-Mass Spectrometry
[0431] RT retention time
[0432] MP melting point
[0433] NMR nuclear resonance spectrum
[0434] TLC thin layer chromatography
[0435] ES Electrospray
[0436] DMAC N,N-Dimethylacetamide
[0437] HRMS High Resolution Mass Spectrometry
[0438] CDI 1,1'-carbonyldiimidazole
[0439] HOBT 1-Hydroxybenzotriazole
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com