Use of AR-A014418 in preparing medicine for preventing and treating nerve degenerative diseases
A neurodegenerative and drug technology, applied in nervous system diseases, drug combinations, pharmaceutical formulations, etc., can solve the problems of unable to prevent neuron loss, uncontrollable disease development, etc., to prevent neuron apoptosis, achieve significant curative effect, inhibit active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
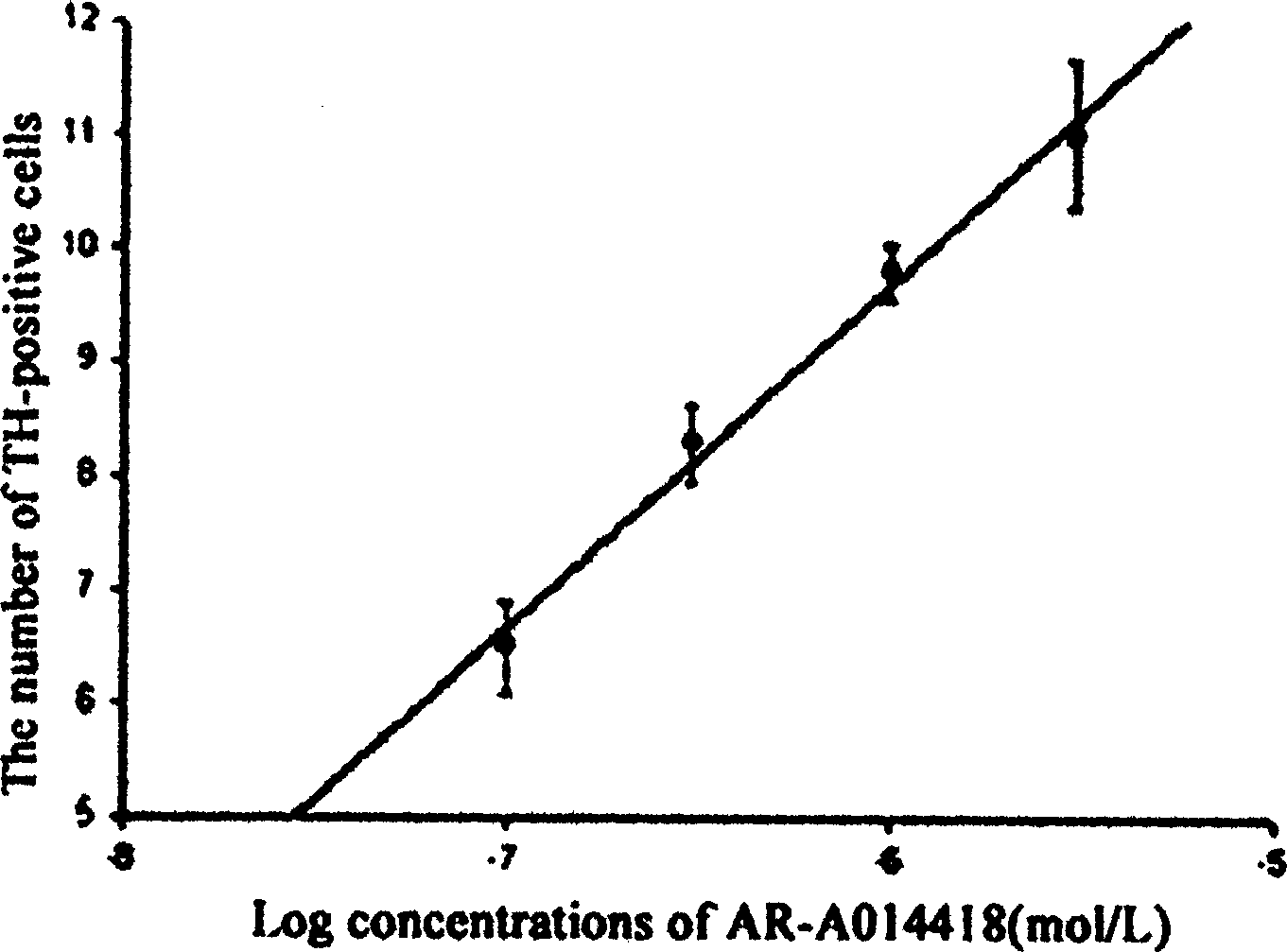
Image
Examples
Embodiment 1
[0123] The preparation of embodiment one injection
[0124] Weigh 10 grams of AR-A014418, dissolve it in 1000 milliliters of water for injection, dissolve it, and filter it with an ultra-microfiltration membrane to prepare an injection solution with a concentration of 1 gram / 100 milliliters (1%), sterilize and dispense.
[0125] For patients with Parkinson's disease and senile dementia: the dosages for subcutaneous injection, intramuscular injection, and slow intravenous injection are all the same, 50-100 mg each time, 2-3 times a day.
Embodiment 2
[0126] The preparation of embodiment two tablet
[0127] Composition: AR-A014418 20g
[0128] Starch 6g
[0129] Citric acid 0.2g
[0130] 10% starch slurry appropriate amount
[0131] 1% magnesium stearate appropriate amount
[0132] 0.2 g of citrate was dissolved in 20 ml of 10% starch slurry. Take the proportioning amount of AR-A014418 and starch, mix evenly, add an appropriate amount of 10% starch slurry containing citric acid to make soft material, pass through a 16-mesh sieve to granulate, dry the wet granules at 40-60°C; granulate with a 16-mesh sieve, After adding an appropriate amount of 1% magnesium stearate, it is compressed to form tablets each containing 100 mg of AR-A014418.
[0133] For patients with Parkinson's disease and Alzheimer's disease: take 0.5-1.0 g orally once a day, and give it in 2-4 times.
Embodiment 3
[0134] The preparation of embodiment three sustained-release capsules
[0135] Composition: AR-A014418 10g
[0136] Calcium hydrogen phosphate 10g
[0137] HPMC4000cp 2g
[0138] HPMC100cp 2g
[0139] EC100cp 0.5g
[0140] Magnesium stearate 0.25g
[0142]The composition and weight percentage contained in the capsule are: AR-A01441840%, calcium hydrogen phosphate 40%, HPMC-4000cp 8%, HPMC100cp 8%, EC100cp 2%, magnesium stearate 1%, talcum powder 1%. The preparation method is as follows: pass the proportioning amount of AR-A014418, calcium hydrogen phosphate, HPMC4000cp, HPMC 100cp and EC 100cp through a sieve of 60 mesh to 100 mesh respectively, put it in a mixer and mix it evenly, and add alcohol with a concentration of 50% to 75% as a moisturizer. Wet agent and stirred to make soft material, pass through 14 mesh to 20 mesh sieve to granulate, dry the granules at 50°C to 60°C, granulate with 14 mesh to 20 mesh sieve, then add magnesium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com