Method for preparing olefin by catalytic oxidation of petroleum hydrocarbon
A technology for catalytic oxidation and petroleum hydrocarbons, applied in the field of low-carbon olefins, can solve problems such as high energy consumption and complex equipment, and achieve the effects of improving process efficiency, reducing energy consumption, reducing regeneration temperature and solvent-oil ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
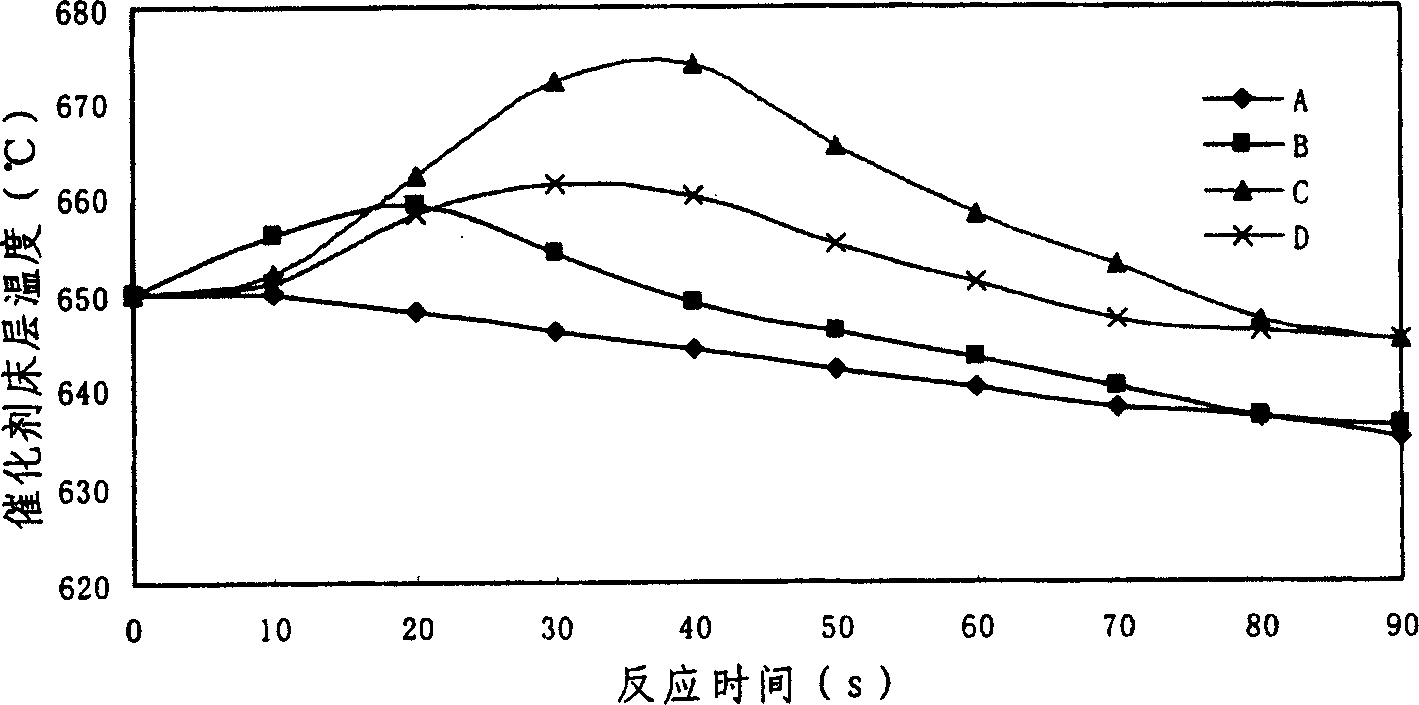
[0018] Fixed bed reactor, the reaction raw material is naphtha, the composition is shown in Table 1. The cracking catalyst is a solid acid catalyst A; the cracking catalyst and three different metal oxide catalysts are uniformly mixed at a volume ratio of 6.5:1 to obtain catalysts B, C and D. The properties of the catalysts are shown in Table 2. The reaction tube has an inner diameter of 6 mm and a tube length of 300 mm. The reaction tube has a jacket outside. When the reaction is carried out, the inside of the jacket is evacuated, and the external heating furnace is closed to stop heating. figure 1 ). The particle size of the catalyst is 40-60 mesh, and the loading amount of the catalyst is 6ml. The reaction carrier gas was pure nitrogen, and the carrier gas flow rate was 22.5ml / min. After 1.5 minutes of reaction, the product passed through the cold trap and collected the gas product by draining the gas. The reaction results are shown in Table 3. Under the action of catalys...
Embodiment 2
[0024] Fixed fluidized bed reactor, the reaction raw material is Daqing atmospheric residue, and the physical and chemical properties of Daqing residue are shown in Table 4. Composite catalyst E is a mixture of solid acid catalyst F (Chinese patent 03141148.7) and metal oxide catalyst G. The physical properties of the catalyst are shown in Table 5. The weight ratio of F and G is 10:1. Under the action of pure solid acid catalyst F and composite catalyst E respectively, the agent-oil ratio is 18:1, the water-oil ratio is 0.3:1, the reaction time is 60 seconds, the reaction temperature is 500°C, 600°C, 650°C, the reaction results See Table 6.
[0025] Density (kg / m 3 )
[0026] catalyst
[0027] Reaction temperature (°C)
[0028] Table 6 shows the results of coupled reactions of cracking reaction and oxidative cracking on a fixed fluidized bed. On the catalyst added with metal oxide, the temperature drop of the catalyst bed is reduced during the...
Embodiment 3
[0030] The composite catalyst is regenerated after reacting in the reactor, the solid acid catalyst is regenerated by burning, and the metal oxide is regenerated by oxidation. The reaction results after catalyst regeneration are shown in Table 7. The catalyst used for the reaction is E, the reaction temperature is 650° C., and other reaction conditions are the same as in Example 2.
[0031] Regeneration times
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com