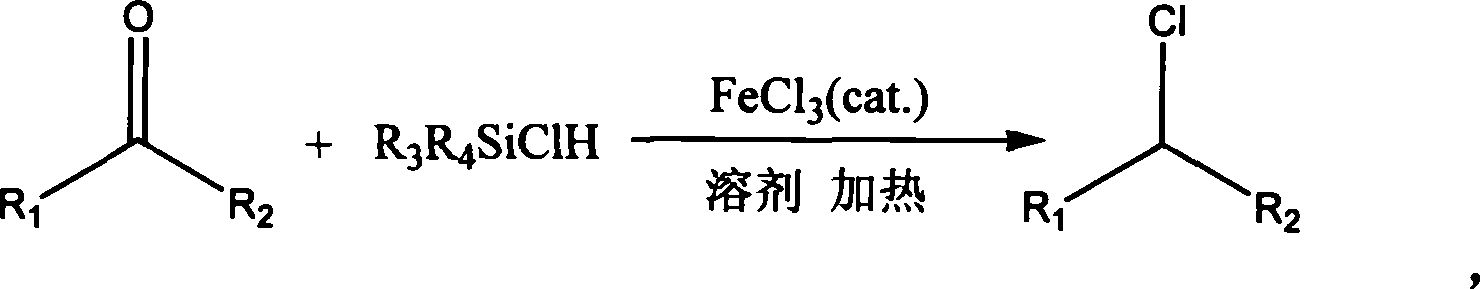
Method for direct chlorination synthesis of chlorohydrocarbon adopting chlorosilane carbonyl compound
A technology for carbonyl compounds and chlorinated hydrocarbons, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of long process flow, cumbersome complexity, increased difficulty, etc., and achieves simple process and good economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the anhydrous FeCl of 0.02mol 3 Put it into the reactor, add ethylene glycol dimethyl ether 30ml, 1mol benzaldehyde and 1mol diphenylchlorosilane, stir well, anhydrous FeCl 3 After basically dissolving, start heating to reflux, control the temperature at 90°C, stir for 2 hours, filter to remove inorganic matter, and distill under reduced pressure to obtain benzyl chloride. The yield was 95.2%.
Embodiment 2
[0024] Embodiment 2: the anhydrous FeCl of 0.5mol 3 Put it into a container, add 30ml of tetrahydrofuran, add 0.5mol of p-chlorobenzaldehyde and 0.5mol of methyldichlorohydrosilane, stir well, anhydrous FeCl 3 After basically dissolving, start heating to reflux, control the temperature at 70°C, stir for 3 hours, filter to remove inorganic matter, and distill under reduced pressure to obtain p-chlorobenzyl chloride. The yield was 93.5%.
Embodiment 3
[0025] Embodiment 3: the anhydrous FeCl of 0.05mol 3 Put it into a container, add 30ml of tetrahydrofuran, add 0.5mol of p-nitrobenzaldehyde and 0.3mol of phenyldichlorosilane, stir well, anhydrous FeCl 3 After basically dissolving, start heating to reflux, control the temperature at 70°C, stir for 4 hours, filter to remove inorganic matter, and distill under reduced pressure to obtain p-nitrobenzyl chloride. The yield was 90.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com