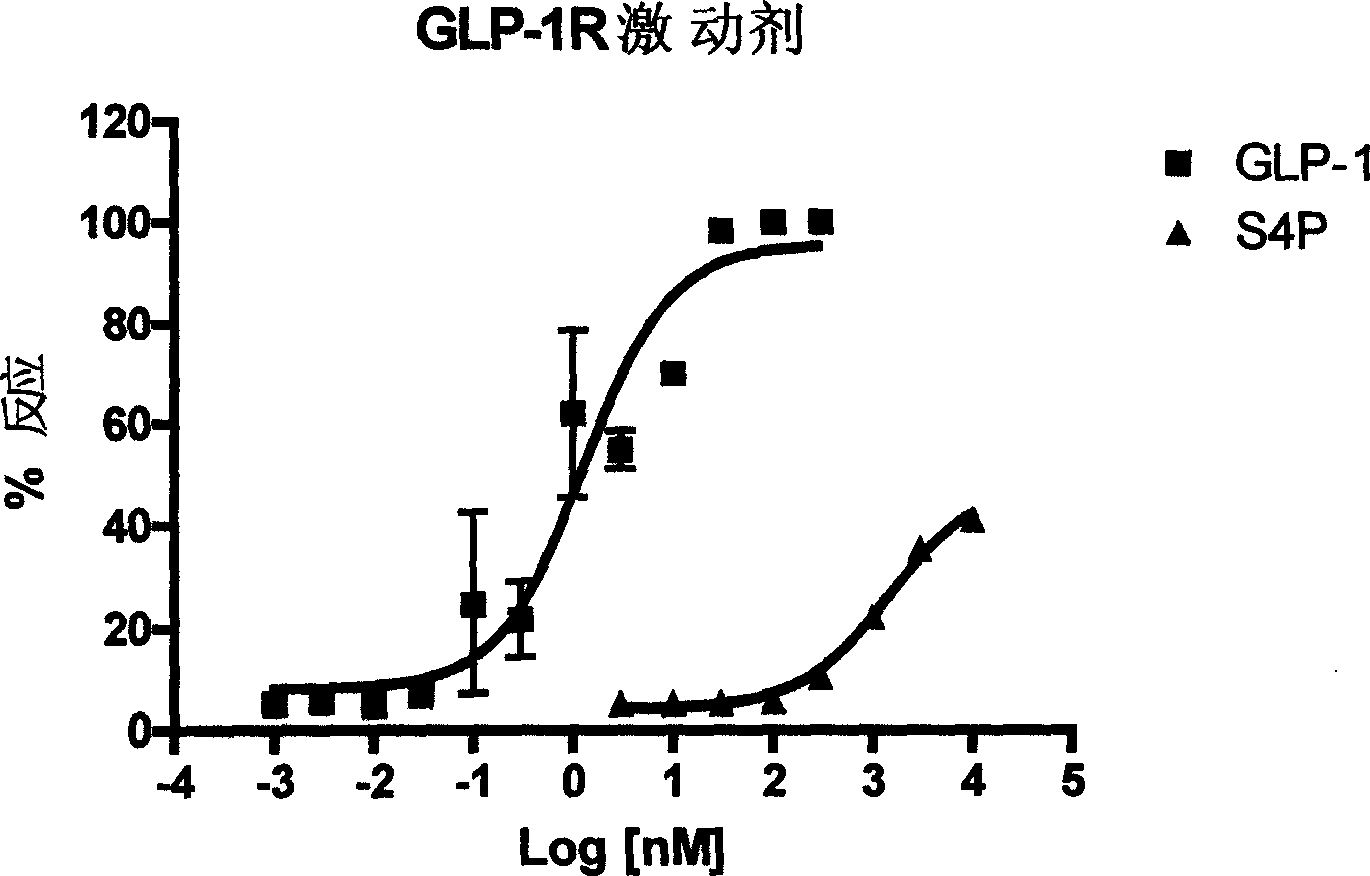
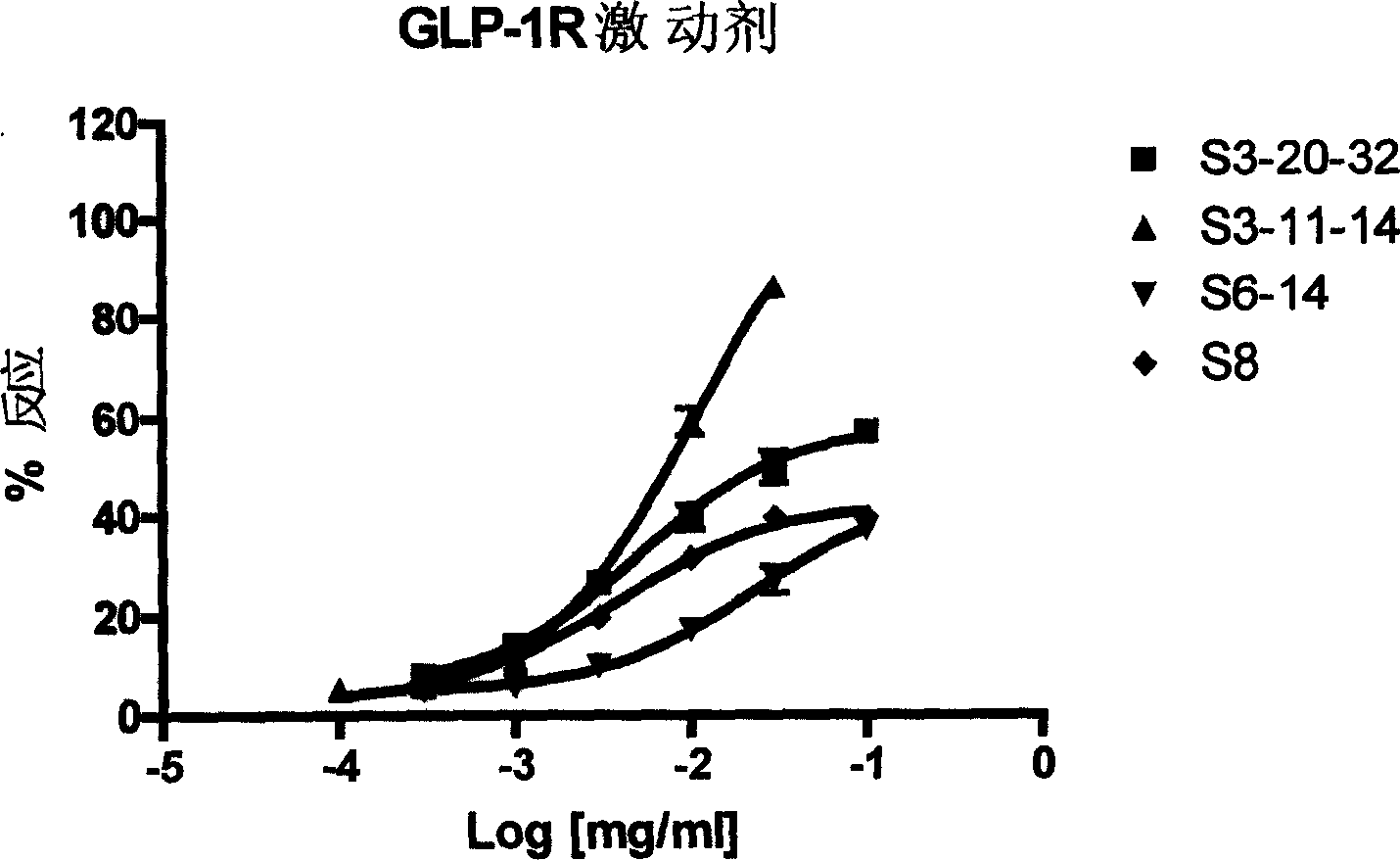
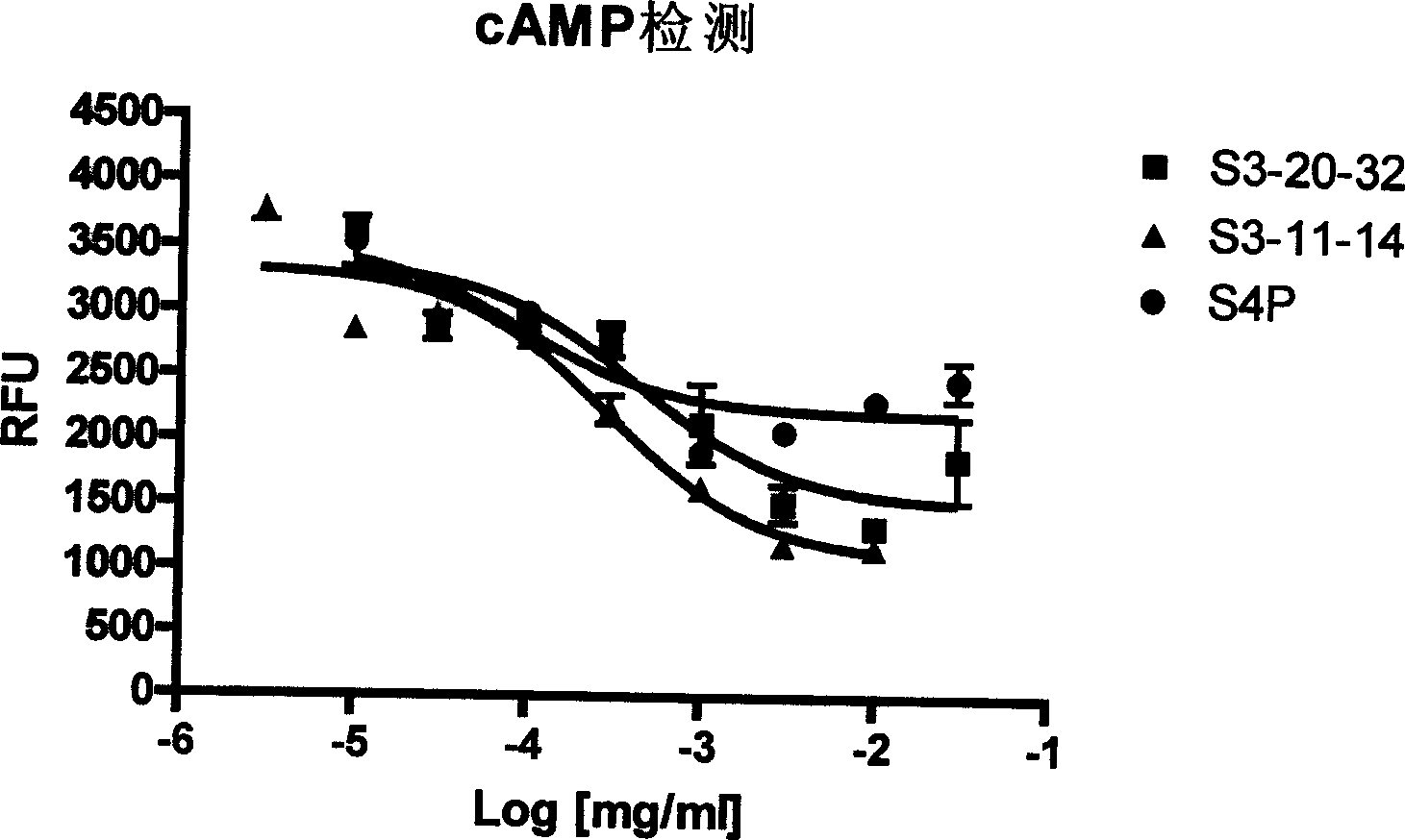
Glucagon-like peptide-1 receptor regulator, its preparation method and uses
A receptor regulator, glucagon technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230] NMR calibration: δH / C 7.26 / 77.0ppm (CDCl3); δH / C 2.50 / 39.51ppm (DMSO-d6).
[0231]
[0232] Compound 1 (1g) was dissolved in an appropriate amount of DMSO, placed under a 150W high-pressure mercury lamp for 3 days of irradiation, 1ml of water was added, and the irradiation was continued for 7-10 days, during which the reaction was detected and tracked by HPLC. After the reaction is completed, the solvent is removed by lyophilization, and the residue is separated by column chromatography [the chromatographic column is ZORBAX SB-C18 (2.1 × 150mm, 3.5 μm), the mobile phase is acetonitrile / water=65: 35, and the flow rate is 0.2ml / min, The detection wavelength is 254nm]. Compound 2 was obtained as light yellow powder solid.
[0233] 1 HNMR (300MHz, DMSO-d6) 10.053 (2H, br.s), 8.630 (2H, br.s), 8.029 (2H, dd, J 1 = 3.6Hz,J 2 = 1.2Hz), 8.090 (2H, dd, J 1 = 4.8Hz,J 2 =1.2Hz), 7.605(4H, d, J=8.4Hz), 7.395(4H, d, J=8.1Hz), 7.31(2H, m), 7.280(2H, br.s), 7.260(2H, m ), 7....
Embodiment 2
[0236]
[0237] Compound 1 (10g) was dissolved in an appropriate amount of DMSO, placed at room temperature under natural light for 30-90 days, lyophilized to remove the solvent, and the residue was separated by HPLC [the chromatographic column was ZORBAX SB-C18 (2.1×150mm, 3.5μm) , the mobile phase is acetonitrile / water=65:35, the flow rate is 0.2ml / min, and the detection wavelength is 254nm], a small amount of light yellow powdery solid compound 2 can be obtained.
Embodiment 3
[0239]
[0240] Compound 1 (20 mg) was dissolved in an appropriate amount of DMSO, 25 mg of benzophenone was added, and placed under a 150w high-pressure mercury lamp for irradiation for 1 day, the solvent was removed by lyophilization, and the residue was separated by column chromatography [the chromatographic column was ZORBAX SB-C18 ( 2.1×150 mm, 3.5 μm), the mobile phase is acetonitrile / water=65:35, the flow rate is 0.2 ml / min, and the detection wavelength is 254 nm] to obtain light yellow powdery solid compound 2.
[0241]In the above reaction example, according to the reaction conditions, the reaction solution is dichloromethane, dichloroethane, water, DMSO or a mixed solvent of DMSO and water, dioxochlorocycline or a mixed solvent of the above solvents. Natural light, light or high-pressure mercury lamps are used to irradiate, the light temperature is 0° to room temperature, and the light time is 1 to 90 days. During the reaction, a catalytic amount of benzophenone is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com