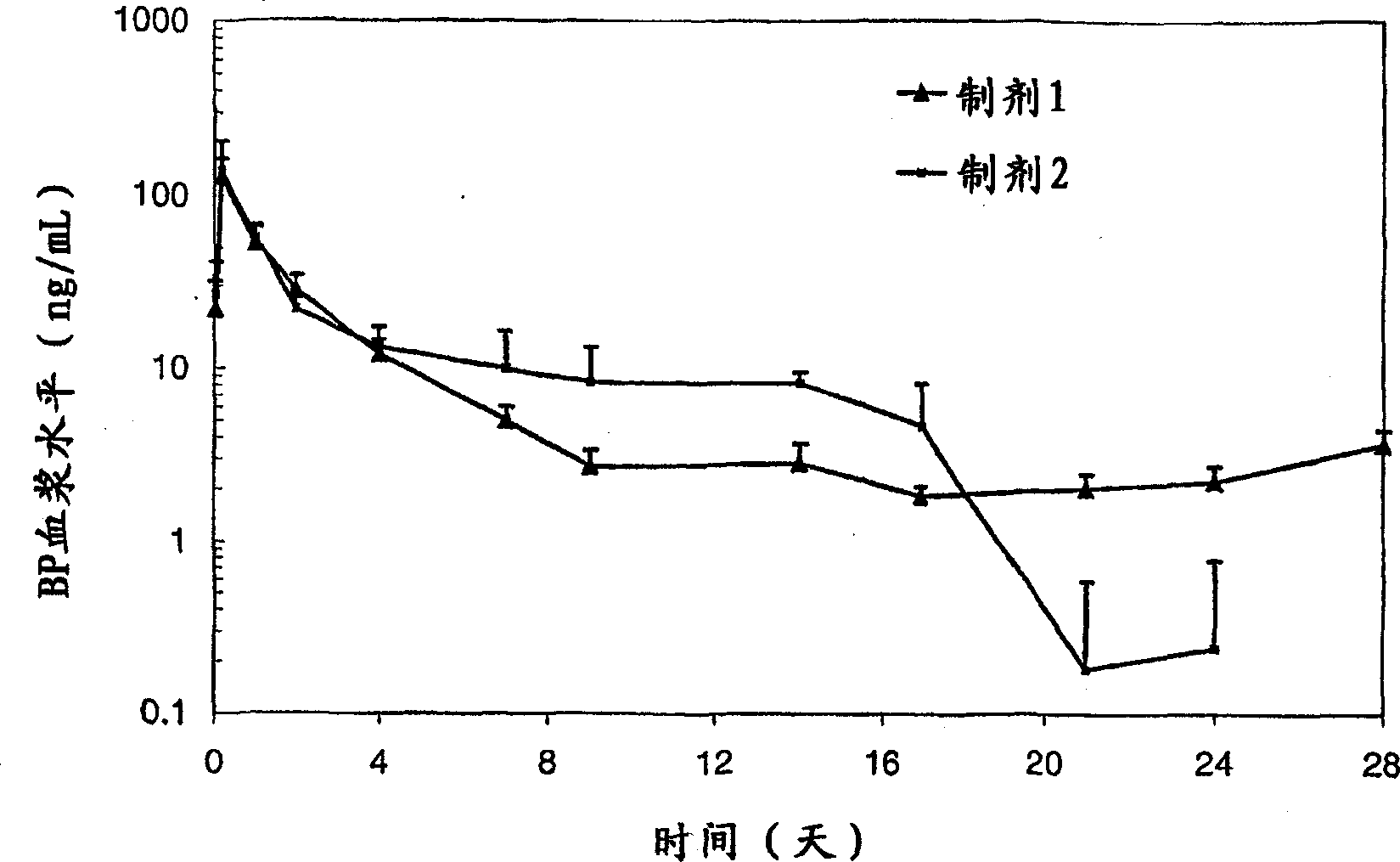
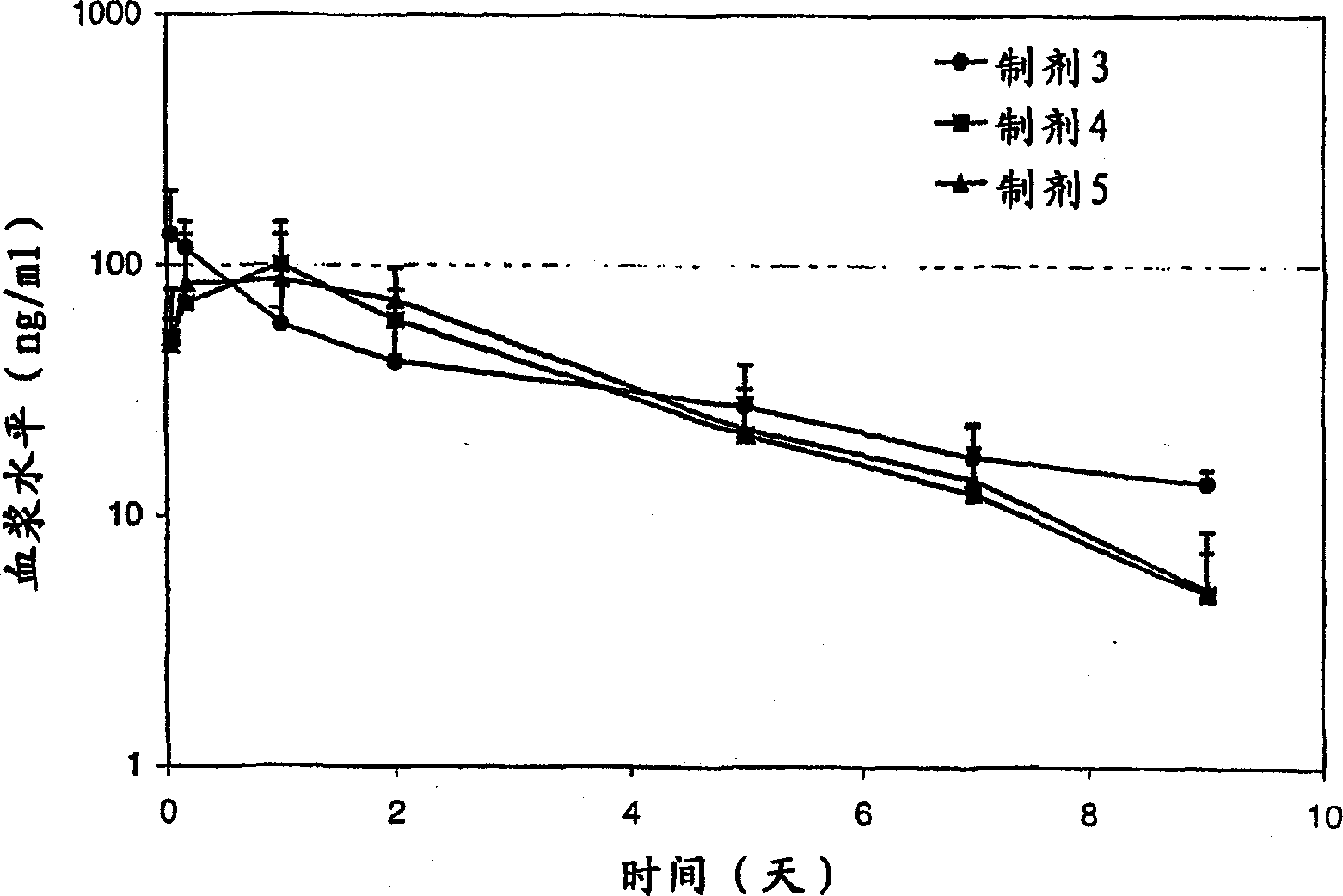
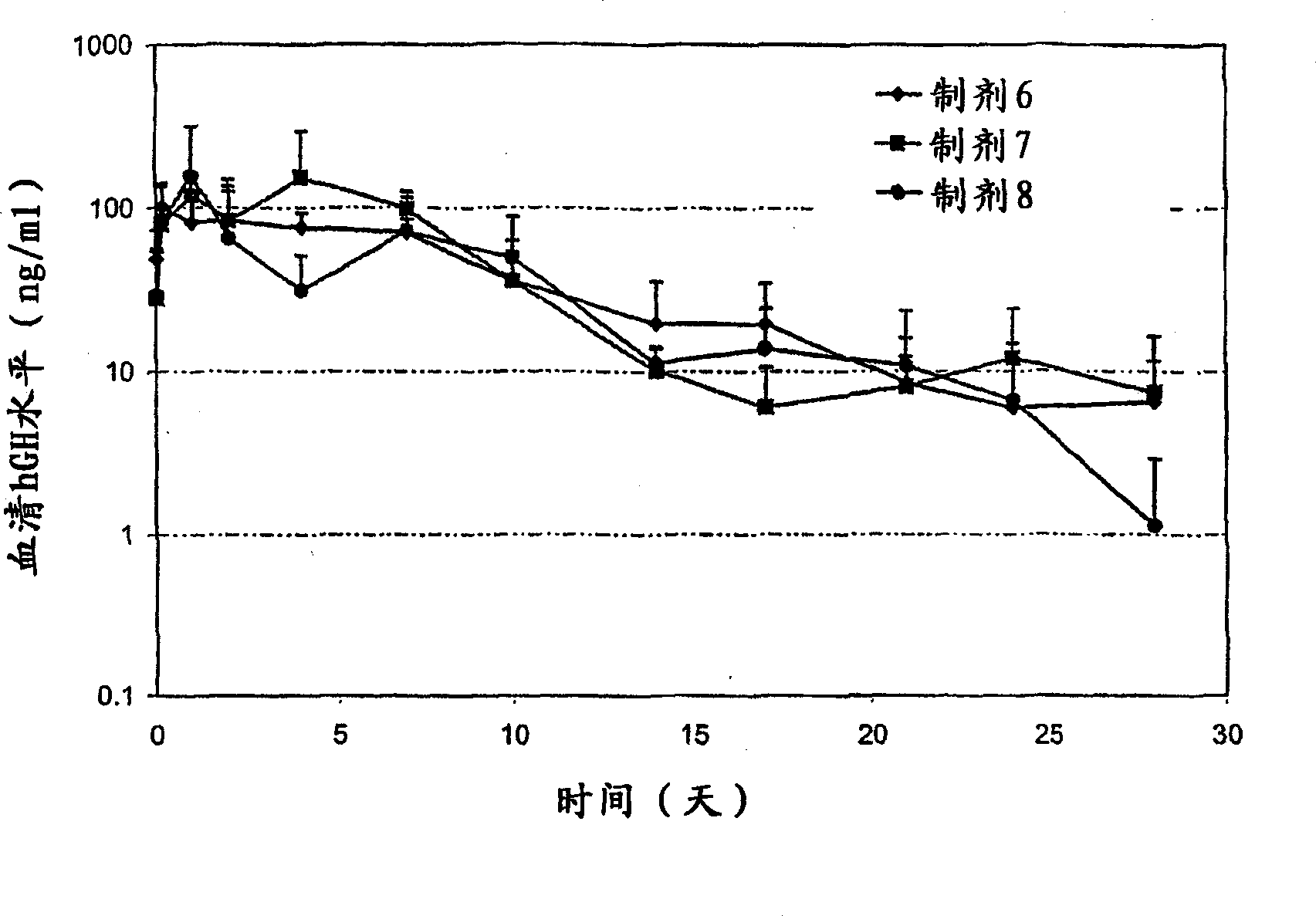
Excipients in drug delivery vehicles
A technology of excipient and vehicle, applied in the field of preparation and administration of the composition, can solve the problems of reducing the overall effectiveness of the dosage form, and the easy deterioration of beneficial components.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] depot gel preparation
[0115] Gel vehicles for use in injectable depot compositions are prepared as follows. Place the glass container on a Mettler PJ3000 top loading balance. Poly(D,L-lactide-co-glycolide) (PLGA) was weighed into a glass container as 50:50 DL-PLG with an intrinsic viscosity of 0.15 (PLGA-BPI, Birmingham Polymers, Inc., Birmingham, AL), and 50:50 RG502 (PLGA RG502). The glass container containing the polymer was tared and the corresponding solvent was added. The percentages for various polymer / solvent combinations are described in Table 1 below. The polymer / solvent mixture was stirred (IKA electric stirrer, IKH-Werke GmbH and Co., Stanfen, Germany) at 250±50 rpm for about 5-10 minutes to obtain a viscous paste-like mass containing polymer particles. The vessel containing the polymer / solvent mixture was sealed and placed in a temperature-controlled incubator equilibrated to 37°C for 1 to 4 days with intermittent agitation, depending on solvent to ...
Embodiment 2
[0118] Bupivacaine base preparation
[0119] Bupivacaine hydrochloride (Sigma-Aldrich Corporation, St. Louis, MO) was dissolved in deionized (DI) water at a concentration of 40 mg / ml (saturated). To this solution was added a calculated amount of sodium hydroxide (1 N solution) to adjust the pH of the final mixture to 10 to precipitate out the BP base. The precipitated product was filtered and further washed with DI water at least three times. The precipitated product was dried under vacuum at about 40°C for 24 hours.
Embodiment 3
[0121] Bupivacaine particle preparation
[0122] Bupivacaine drug particles were prepared using bupivacaine hydrochloride (Sigma-Aldrich Corporation, St. Louis, MO) or bupivacaine base and hydrochloride prepared according to Example 2 as follows. Bupivacaine was ground and then sieved using a 3" stainless steel sieve. Typical ranges included 25 μm to 38 μm, 38 μm to 63 μm, and 63 μm to 125 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com