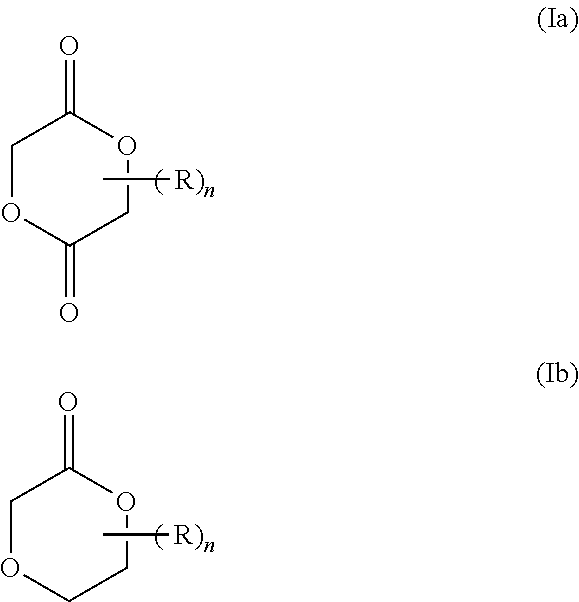
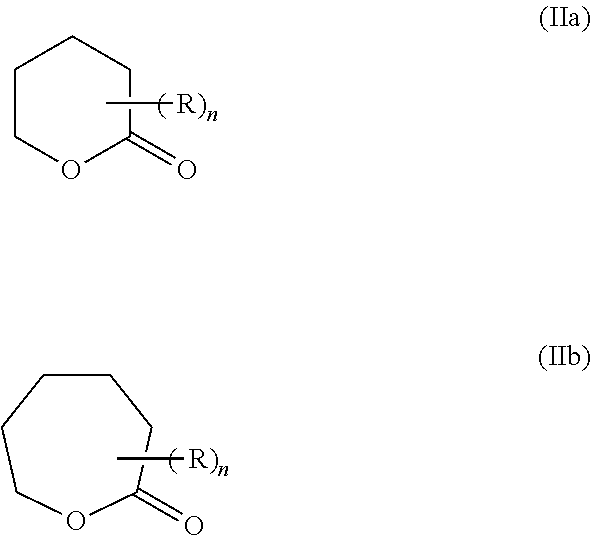
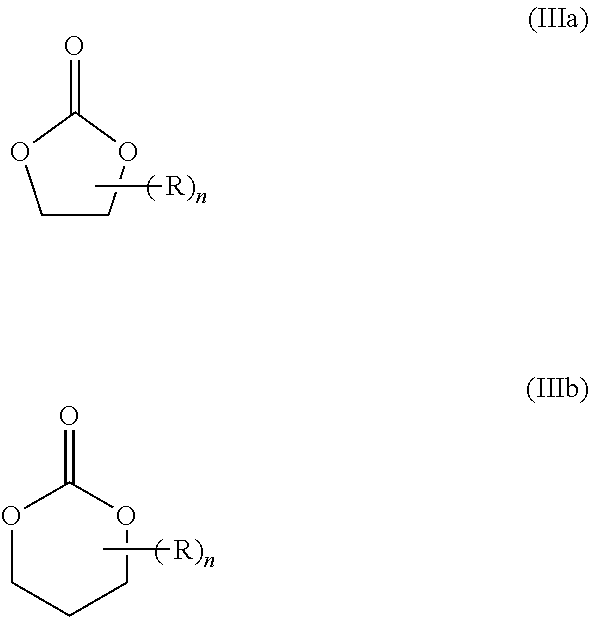
Cyclic ester dual cure resins for additive manufacturing
a technology of additive manufacturing and cyclic ester, which is applied in the direction of manufacturing tools, prosthesis, other domestic objects, etc., can solve the problems of general limitation of the material used in such apparatuses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-4
[0116]Conventionally, UV curable bioabsorbable materials are based on di-methacrylate / acrylate terminated crosslinkers with bioabsorbable oligomers as the linkage. Such linkage may be polyethylene glycol (PEG), polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), polydioxanone (PDO), poly(4-hydroxybutyrate) (P4HB), and their copolymers. Although this approach works in producing UV curable bioabsorbable materials, the resulting material can have a high fraction of methacrylate / acrylate backbone which is generally considered non-bioabsorbable. Upon complete degradation of the bioabsorbable linkages, carboxylic acid groups are produced on methacrylate / acrylate backbone, making it soluble in aqueous environment. However, if the molecular weight of methacrylic / acrylic acid is higher than 30,000, the backbone becomes difficult to dissolve in an aqueous environment.
[0117]An orthogonal curing system can potentially mitigate these issues. In addition to the UV curable netw...
example 1
[0120]
[0121]An alcohol is used as the initiator. The ring-opening polymerization is catalyzed by Tin(II) Octoate at elevated temperatures (>60° C.). The reason for curing above 60° C. is that polycaprolactone is crystalline and melts slightly below 60° C. To obtain a significant polymerization rate, the product must be in liquid state.
example 2
[0122]
[0123]An alcohol is used as the initiator. The ring-opening polymerization is catalyzed by a basic organocatalyst, commonly 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD), N-methyl-TBD (MTBD), and 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU). TBD was shown to be most effective in catalyzing ring-opening polymerization of caprolactone. See Lohmeijer et al., “Guanidine and Amidine Organocatalysts for Ring-opening Polymerization of Cyclic Esters,”Macromolecules 2006, 39, 8574-8583.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com