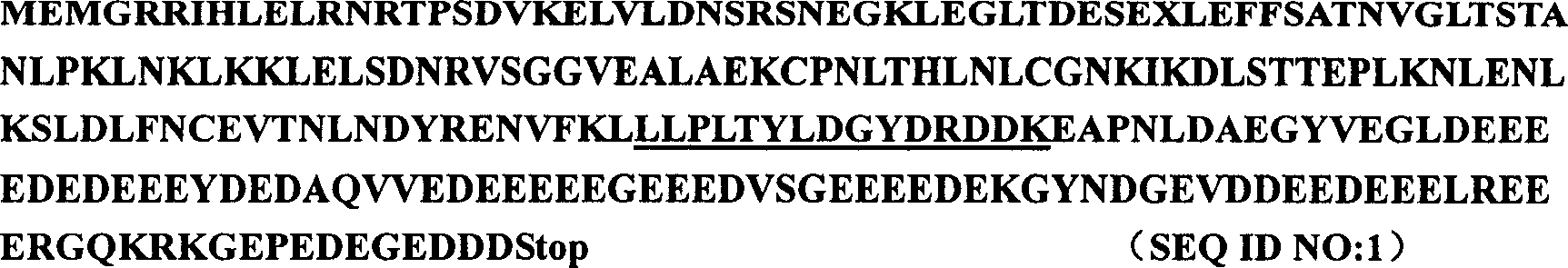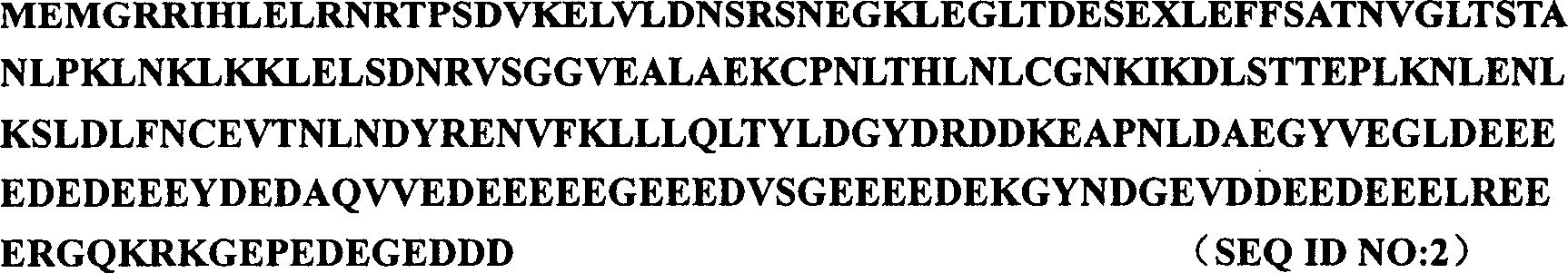Liver regenerated factor and its use
A technology for liver regeneration, reason, applied in the field of biological products and protein drugs, which can solve problems such as lack of stimulatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Production and preparation of HPPCn and its function of promoting liver cell proliferation and liver regeneration
[0024] Using DEAE-cellulose, Source15Q and other chromatography methods, SDS-PAGE segmented recovery, and MALDY-TOF and Q-TOF mass spectrometry techniques, a protein that can specifically promote hepatocyte proliferation and liver regeneration was purified and identified from newborn calf liver Factor, namely HPPCn. The human HPPCn cDNA sequence was obtained by screening the human fetal liver cDNA library. Add BamH I and Xhol I restriction sites at both ends of the gene sequence, construct it into the prokaryotic expression vector PBV220, and obtain the PBV220-HPPCn plasmid. It was transformed into Escherichia coli, and its expression was induced at 42°C. After ion exchange and gel filtration, the recombinant HPPCn with a purity of more than 95% is obtained, and its amino acid sequence is as follows: figure 1 shown.
[0025] 3 The proliferat...
Embodiment 2
[0029] Example 2 HPPCn to CCl 4 Protective effect of induced acute liver injury
[0030] Thirty Balbc mice in good condition were injected with 1ml / kg CCl 4 After that, they were randomly divided into 3 groups: Group I was given intravenous injection of 2.5 mg / kg HPPCn; Group II was given intravenous injection of 5 mg / kg HPPCn; Group III was given intravenous injection of normal saline. Inject every 12 hours, and observe the changes of ALT, AST and LDH in the blood after 48 hours. The results showed that the AST, ALT and LDH values in serum of 48hr mice after injury were all significantly increased, but the AST, ALT and LDH values of the mice in the HPPCn treatment group were significantly lower than those in the normal saline group (results shown in Figure 6), indicating that HPPCn to CCl 4 The induced acute liver injury has a protective effect.
Embodiment 3
[0031] Example 3 Inhibition of tumor cell growth in HPPCn cells
[0032] Construct HPPCn into the eukaryotic expression vector pEGFP-N1 plasmid, and transfect it into the human liver cancer cell line SMMC7721. After culturing for 36 hours, they were fixed with 4% paraformaldehyde and 70% ethanol, stained with PI, and FACS The changes in the cell cycle were detected, and the results showed that the transfected liver cancer cells showed obvious G0 / G1 phase arrest, and the proportion of cells in the G2 / M phase was significantly less than that of the control cells, suggesting that endogenous HPPCn had obvious growth inhibition on liver cancer cells (See Table 3).
[0033] cell
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com