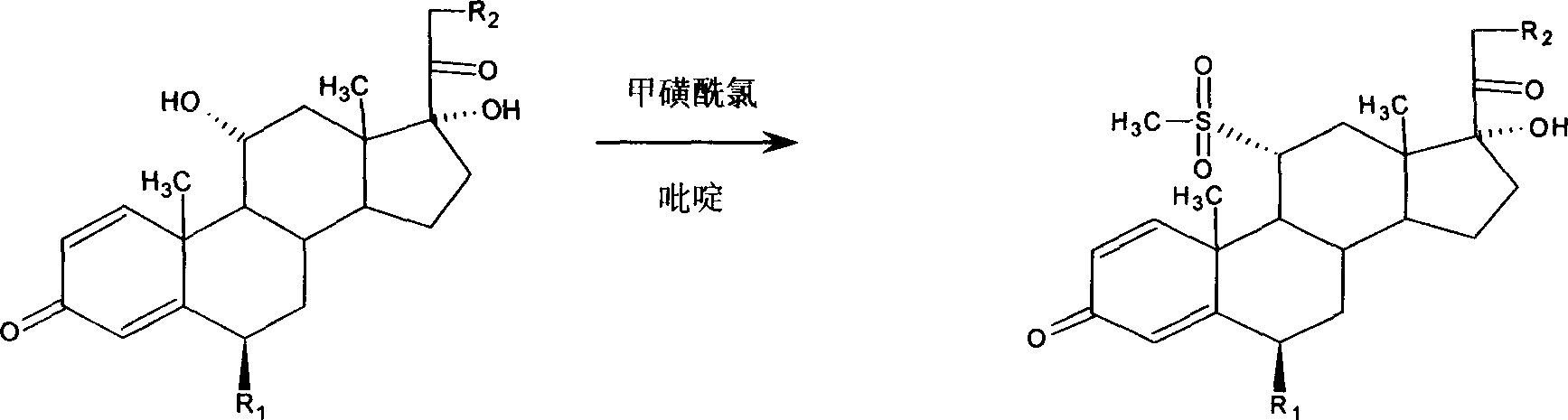
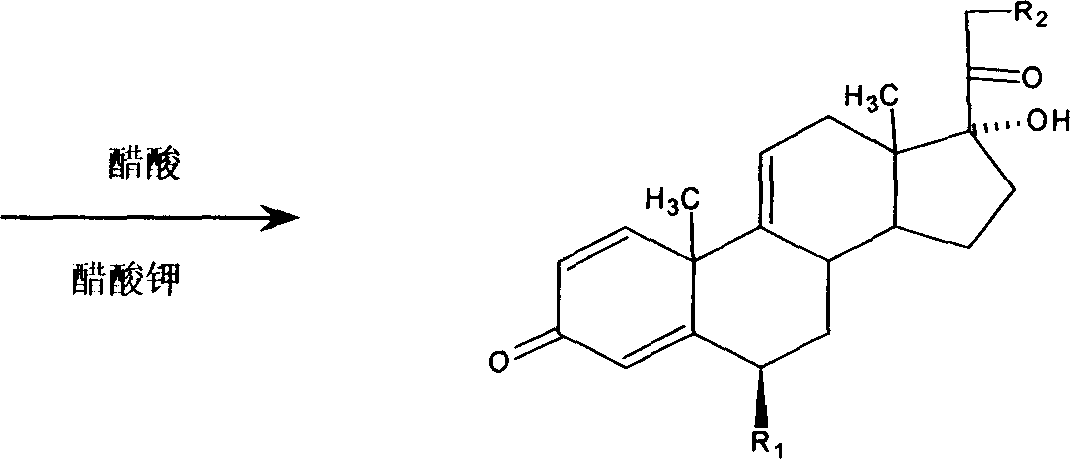
Preparation method for 9,11-de-esterification of metllylpredllisolone series products
A technology for methylprednisolone and series products, which is applied in the field of methylprednisolone series products 9,11—deesterification production technology, and can solve the problem of destroying the structure of the target product, fluctuation of the reaction reflux temperature, and low quality and yield of the main product. and other problems to achieve the effect of reducing the degree of damage, stable reflux temperature, and improving quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: react with dehydrogenate 10g and a certain amount of methanesulfonyl chloride, after completion of the reaction, get sulfonylate wet product 16.7g, react with 60g acetic acid, 25-30g sodium acetate and sulfonylate, heat up to Fully reflux, the reflux temperature is 115°C, after the reaction is completed, dilute, filter, and dry to obtain 8.4 g of methylprednisolone 9,11-desesterified product, the content is 82.2%, and the yield is 84.0%.
Embodiment 2
[0021] Embodiment 2: react with dehydrogenate 10g and a certain amount of methanesulfonyl chloride, after completion of the reaction, get sulfonylate wet product 16.5g, react with 60g acetic acid, 25-30g sodium acetate and sulfonylate, heat up to Fully reflux, add 6.5ml of temperature-controlled water, the reflux temperature is 109°C, after the reaction is completed, dilute, filter, and dry to obtain 8.84g of methylprednisolone 9,11-desesterified product, the content is 86.2%, and the yield is 88.0%.
Embodiment 3
[0022] Embodiment 3: react with dehydrogenate 10g and a certain amount of methanesulfonyl chloride, after completion of the reaction, get sulfonylate wet product 16.9g, react with 60g acetic acid, 25-30g sodium acetate and sulfonylate, heat up to Fully reflux, add 7.5ml of temperature-controlled water, the reflux temperature is 107.5°C, after the reaction is completed, dilute, filter, and dry to obtain 8.75g of methylprednisolone 9,11-desesterified product, the content is 86.7%, and the yield is 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com