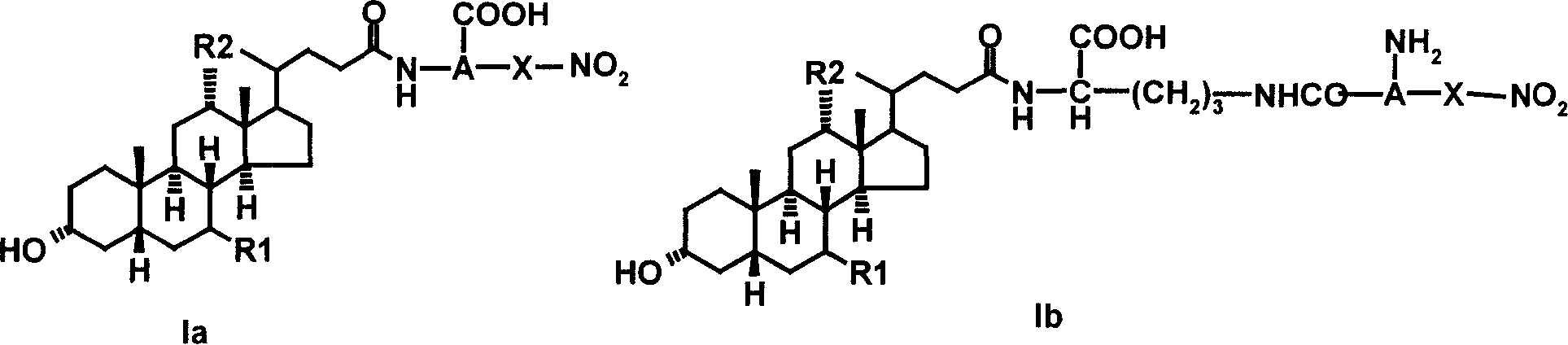
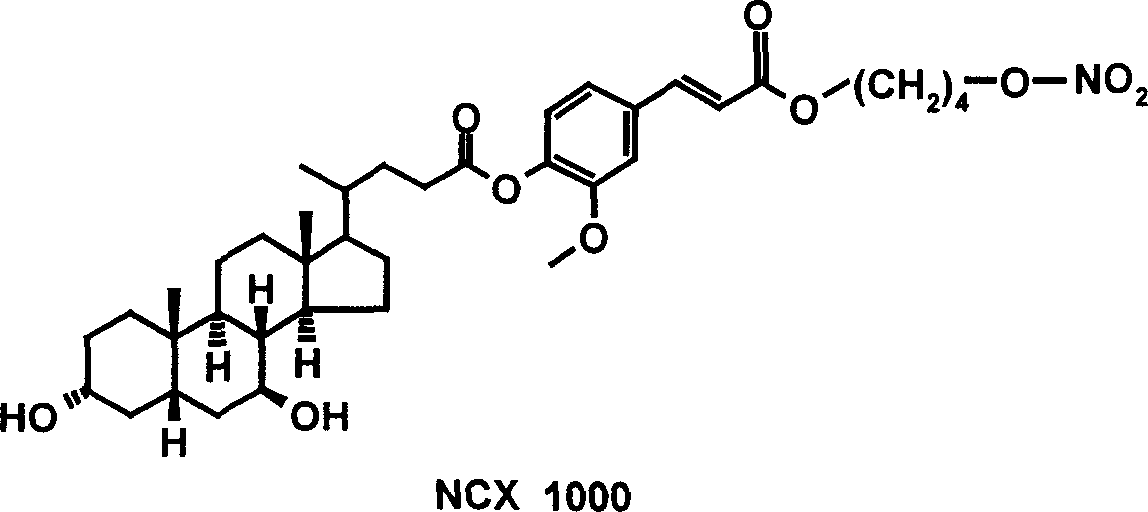
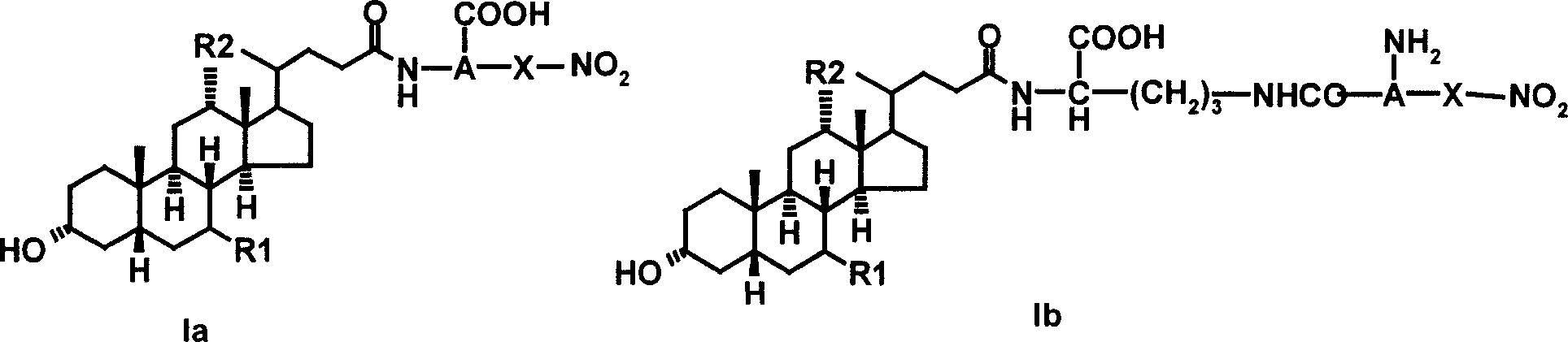
Bile acid derivative and pharmaceutical use thereof
A bile acid and bile acid technology, applied in the preparation of bile acid nitrate derivatives and their pharmaceutically acceptable salts, in the field of medicine for diseases, can solve the problem of drug loss of targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1.N α - Preparation of ursodeoxycholoyl-(3-O-nitro-L-serine) (Ia-1)
[0060] 1.1 Synthesis of 3-O-nitro-L-serine nitrate
[0061] Take 25 ml of fuming nitric acid and put it into a three-necked flask, and cool it to -10°C in an ice-salt bath; add 5 grams of serine to the reaction solution in batches, control the temperature of the reaction solution below 0°C, and react for 30 minutes under stirring. After the reaction was completed, the reaction liquid was dropped into 100 ml of diethyl ether to precipitate a precipitate, which was collected by filtration, thoroughly washed with diethyl ether, and dried to obtain 7.9 g of a white solid with a melting point of 85-88°C and a yield of 78%. IR (film, cm -1 ): 3398, 3005, 1648, 1570, 1384, 1285, 985, 845, 757, 643. 1 H-NMR (DMSO-d 6 ): 8.53 (br s, 3H); 4.98 (q, 1H); 4.85 (q, 1H); 4.50 (br s, 1H). MS (FAB m / e): 211.9 (M+HNO 3 -1), 299.1(2M-1).
[0062] 1.2N α -Synthesis of ursodeoxycholoyl-(3-O-nitro-L-serine)...
Embodiment 2
[0064] Example 2.N α - Preparation of ursodeoxycholoyl-(3-O-nitro-D-serine) (Ia-2)
[0065] Using D-serine instead of L-serine, referring to the method of Example 1.1, 3-O-nitro-D-serine nitrate was obtained, yield: 72%, melting point: 87-91°C. 1 H-NMR (DMSO-d 6 ): 8.49 (brs, 3H) 5.01 (br m, 2H) 4.68 (br m, 1H). MS (FAB m / e): 151.1 (M+1) 301.0.1 (2M+1).
[0066] Using 3-O-nitro-D-serine nitrate instead of 3-O-nitro-L-serine nitrate, refer to the method of Example 1.2 to obtain the target compound Ia-2. Yield: 74%, melting point: 133-136°C. 1 H-NMR (DMSO-d 6 ): 13.16(br m, 1H); 8.38(d, 1H, J=7.0Hz); 4.84(m, 1H); 4.68(m, 2H); 2.16(br m, 2H); 1.99(s, 3H) ; 1.95-0.84 (brm, steroidal CH 2 and CH); 0.89 (d, 3H, J = 8.1 Hz); 0.61 (s, 3H).
Embodiment 3
[0067] Example 3.N α - Preparation of Choyl-(3-O-nitro-L-serine) (Ia-3)
[0068] Using cholic acid instead of ursodeoxycholic acid, referring to the method of Example 1.2, the target compound Ia-3 was obtained. Yield: 71%, melting point: 133-136°C. 1 H-NMR (DMSO-d 6 ): 13.11(br m, 1H); 8.38(d, 1H, J=7.2Hz); 4.81(brd, 1H); 4.69(m, 2H); 3.78(s, 1H); 3.61(s, 1H); 3.39 (q, 2H); 3.18 (br m, 1H); 2.23-1.07 (br m, steroidal CH 2 and CH); 0.93 (d, 3H, J = 5.9 Hz); 0.81 (s, 3H); 0.58 (s, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com