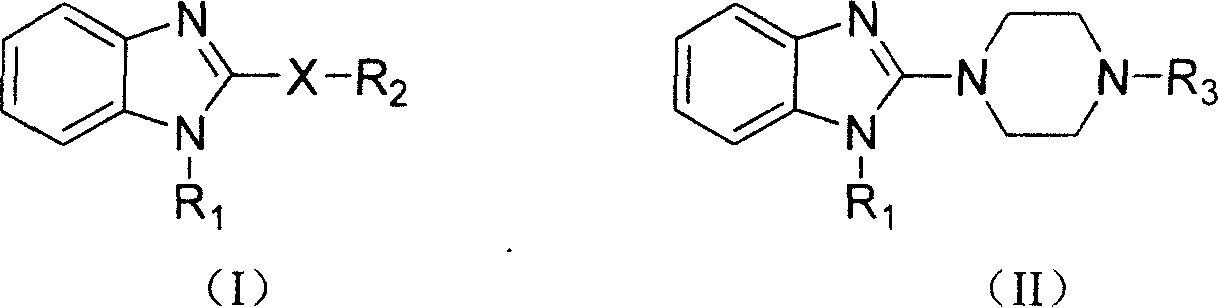
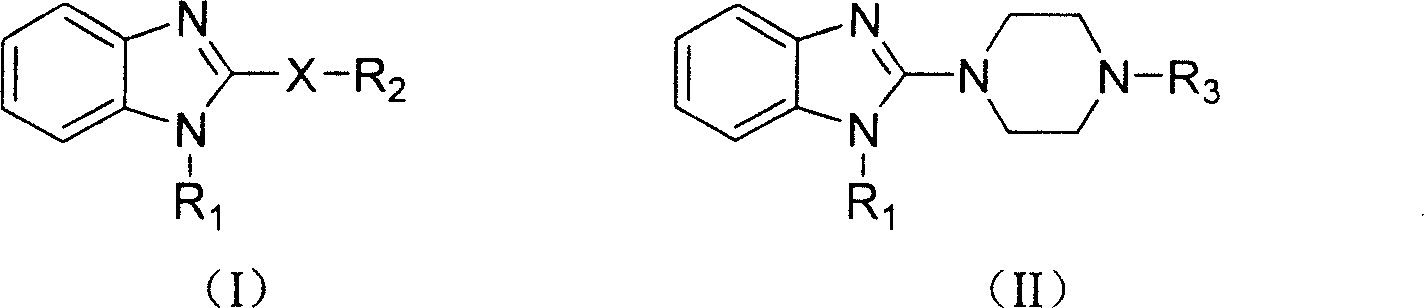
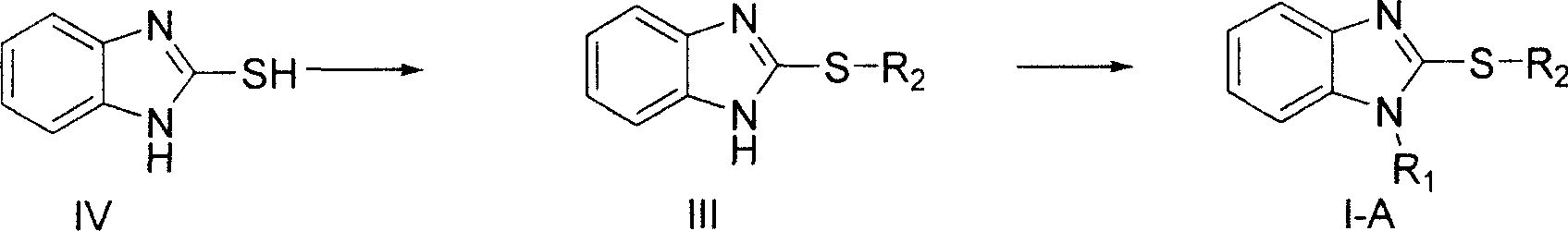
Benzimidazole compound and its preparing method and use in medicine production
A technology of benzimidazole and compound, applied in the field of drug synthesis, can solve problems such as weakened effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: 1-Benzyl-N-p-tolyl-1H-benzimidazol-2-amine
[0067]
[0068] (1) Synthesis of intermediate 1H-benzimidazolone: Add 6.00 g (0.10 mol) of urea and 50 ml of n-amyl alcohol to 5.40 g (0.05 mol) of o-phenylenediamine, and stir and reflux for 10 hours. Cool to room temperature, filter out, recrystallize with absolute ethanol, and dry under infrared light to obtain 5.12 g of light yellow flaky crystals (1H-benzimidazolone), m.p.: 320-322°C, yield: 76.42%.
[0069] (2) Synthesis of intermediate 2-chloro-1H-benzimidazole: add 40mlPOCl to 5.36g (0.04mol) of 1H-benzimidazolone 3 After stirring and refluxing for 3 hours, dry HCl gas was passed into the reflux state for 3 hours. After concentrating the reaction solution, add 40 ml of distilled water while stirring in an ice-water bath to obtain a light yellow turbid liquid, which is filtered, and the filtrate is adjusted to a pH of 8-9 with concentrated ammonia water, and solids are precipitated, and refrigerated ov...
Embodiment 2
[0075] Example 2: 1-Benzyl-N-m-tolyl-1H-benzimidazol-2-amine
[0076]
[0077] By the method of step (4) in Example 1, replace p-methylaniline with m-methylaniline, and 1-benzyl-2-chloro-1H-benzimidazole (according to Example 1 steps (1)~(3) Preparation, the same below) reaction, the title compound was obtained as white powder 0.55g. m.p.: 182-184°C, yield: 87.86%.
[0078] IR(KBr), cm -1 : 3439, 3379, 3037, 1617, 1527, 1492, 1251, 857, 780, 735
[0079] EI-MS: m / z313: m / z222: m / z207(BasePeak):
[0080] 1 H-NMR (DMSO-d6), δ: 8.96 (s, 1H, -NH-), 7.70 (d, 1H, J=8.16Hz, Ar-H), 7.64 (s, 1H, Ar-H), 7.41 (dd, 1H, J=0.71, 7.65Hz, Ar-H), 7.14-7.33(m, 7H, Ar-H), 6.94-7.04(m, 2H, Ar-H), 6.76(d, 1H, J =7.42Hz, Ar-H), 5.53(s, 2H, -NCH 2 ph), 2.30(s, 3H, phCH 3 )ppm.
Embodiment 3
[0081] Example 3: 1-Benzyl-N-(3,4-dimethyl)phenyl-1H-benzimidazol-2-amine
[0082]
[0083]According to the method of step (4) in Example 1, 3,4-dimethylaniline was used instead of p-methylaniline, and 1-benzyl-2-chloro-1H-benzimidazole was reacted to obtain the title compound as a white flocculent Solid 0.31 g. m.p.: 178-179°C, yield: 61.16%.
[0084] IR(KBr), cm -1 : 3481, 1617, 1523, 1251, 819, 739, 713
[0085] EI-MS: m / z327: m / z236: m / z221(Base Peak):
[0086]
[0087] 1 H-NMR (DMSO-d6), δ: 7.63 (dd, 1H, J=2.25, 2.34Hz, Ar-H), 7.53 (d, 1H, J=1.99Hz, Ar-H), 7.38(d, 1H , J=7.58Hz, Ar-H), 7.28-7.33(m, 2H, Ar-H), 7.23-7.26(m, 1H, Ar-H), 7.14-7.20(m, 3H, Ar-H), 6.99-7.08(m, 2H, Ar-H), 6.94-6.99(t, 1H, Ar-H), 5.51(s, 2H, -NCH 2 ph), 2.21(s, 3H, phCH 3 ), 2.17 (s, 3H, ph-CH 3 )ppm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com