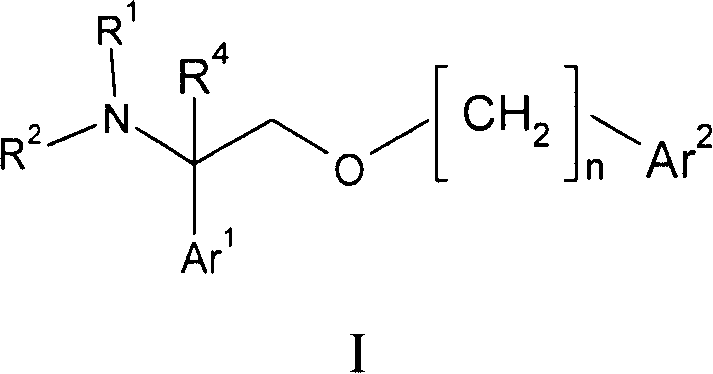
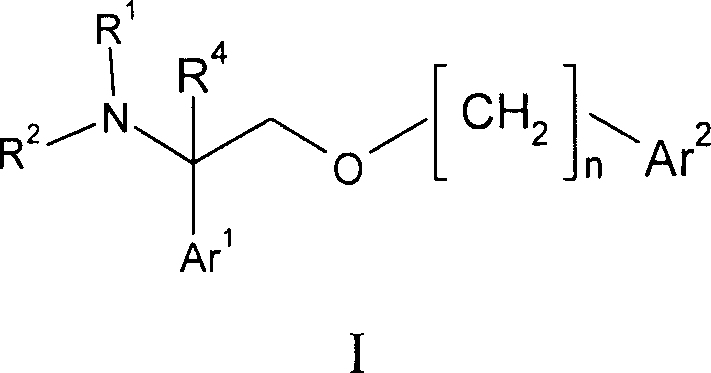
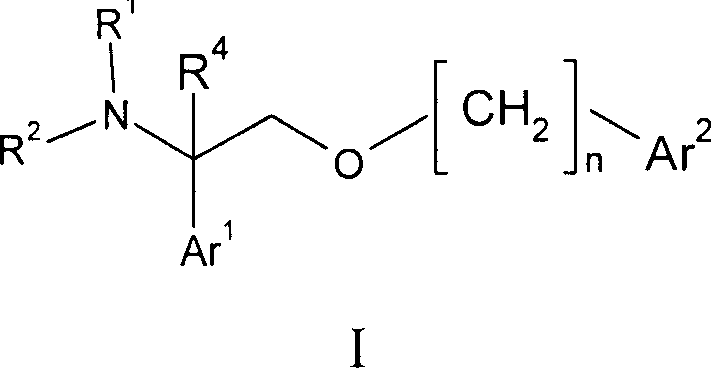
2- ( arylalkoxy ) -1- phenylethylamine derivatives as nk1 antagonist and 5-serotonin reuptake inhibitors
An alkoxy, alkyl technology, applied in the field of 2-(arylalkoxy)-1-phenethylamine derivatives as NK1 antagonists and serotonin reuptake inhibitors, can solve delays, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: 4-{[2-(3-chloro-4-fluorophenyl)-2-piperazin-1-ylethoxy]methyl}-3-methoxy-2-naphthalenecarbonitrile
[0096]4-{1-(3-chloro-4-fluorophenyl)-2-[(3-cyano-2-methoxy-1-naphthyl)methoxy]ethyl}piperazine-1-carboxy Acid tert-butyl ester (100 mg, 0.18 mmol) was dissolved in DCM (2 mL) and TFA (2 mL) was added. After 2 hours the volatiles were removed under reduced pressure. The residue was dissolved in EtOAc (20 mL) and washed with saturated aqueous sodium bicarbonate (20 mL). The organic phase was dried over sodium sulfate, filtered through a pad of celite, and the volatiles were removed under reduced pressure. The residue was chromatographed on silica gel (MeOH: 0-5% 2M NH in DCM 3 ) to afford the title compound (81.6 mg, 79%) as an off-white solid. To a solution of the title compound in methanol was added citric acid (1.0 equiv) to form the citrate salt. Concentration under reduced pressure afforded the desired salt form of the product as an off-white powder. ...
Embodiment 2-29
[0103]
[0104]
[0105]
[0106]
[0107]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com