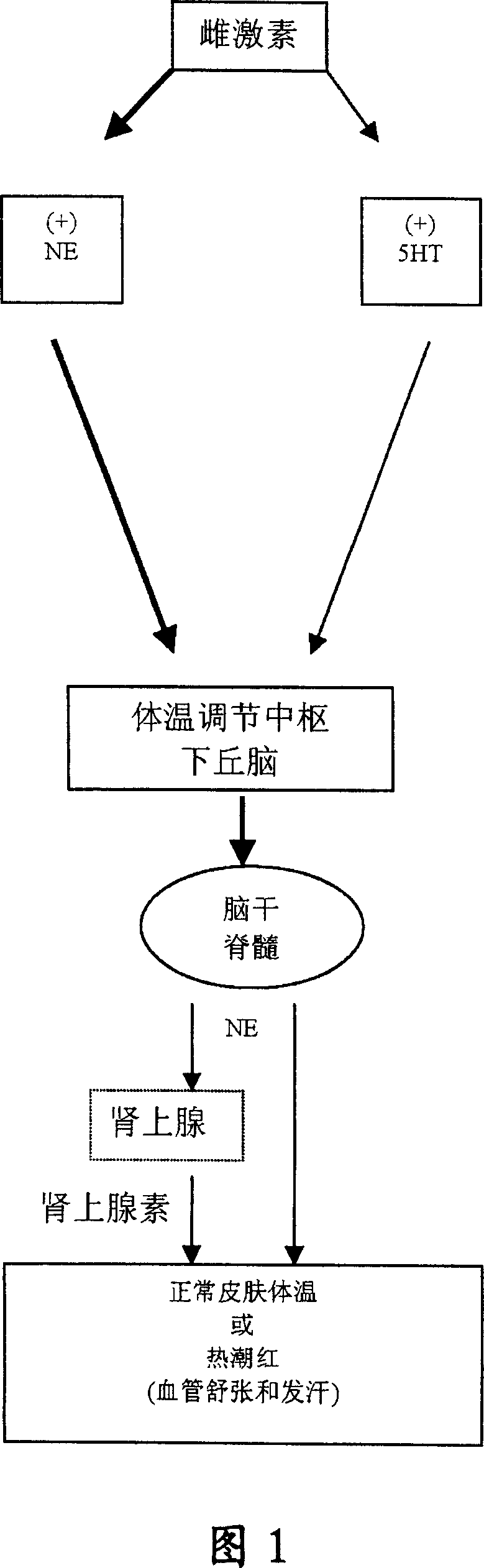
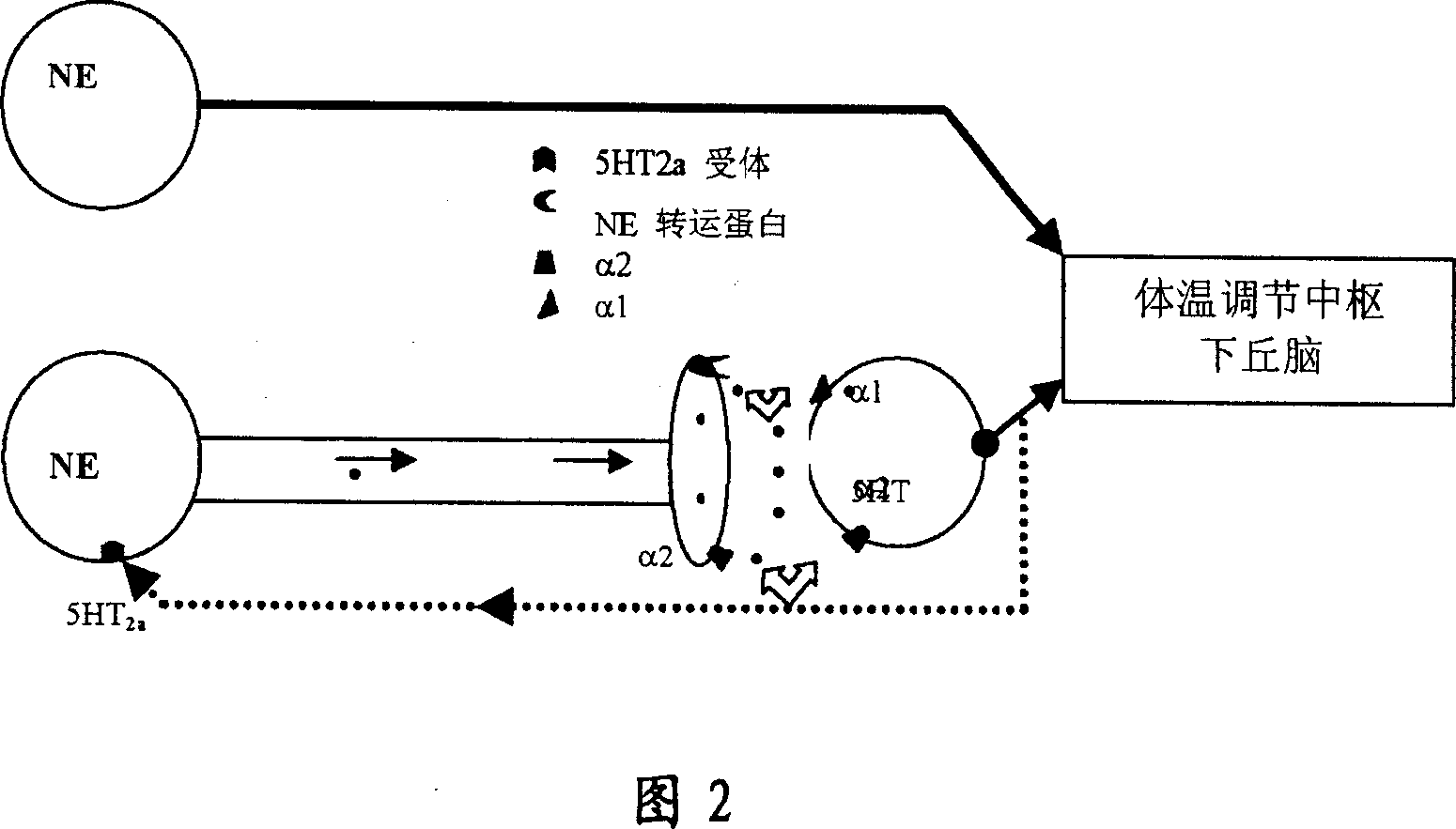
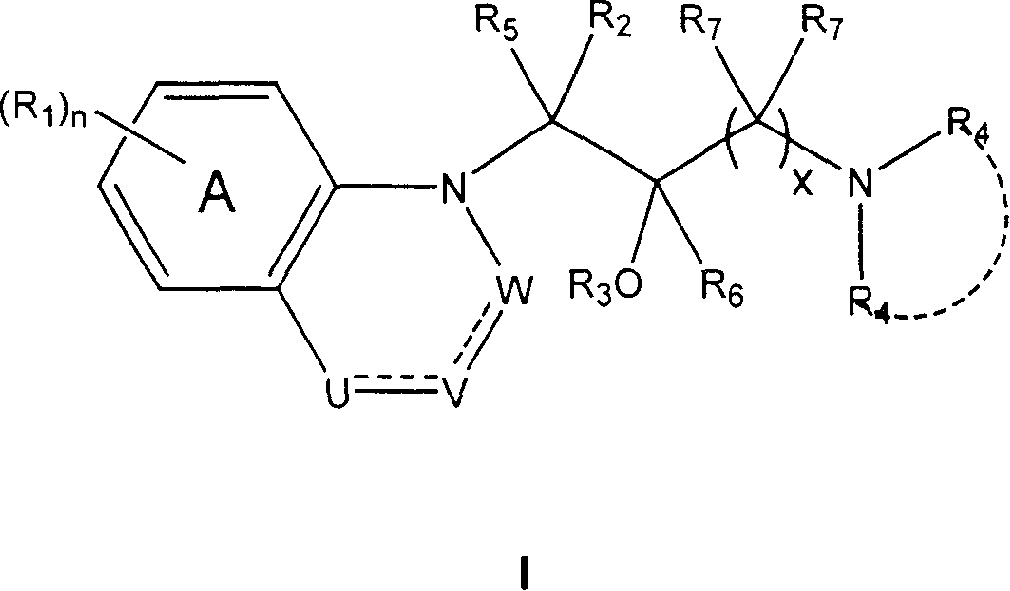
Heterocyclic phenylaminopropanol derivatives as modulators of the monoamine reuptake for the treatment of vasomotor symptoms (VMS)
A technology of methylamino and phenylpropane, which can be used in drug combinations, medical preparations containing active ingredients, diseases, etc., and can solve problems such as non-recommendation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0252] Example 1: (1RS, 2SR)-1-(1H-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenylpropan-2-ol dihydrochloride Salt
[0253]
[0254] Step 1: A mixture of indole (2.34 g, 20 mmol) and crushed solid potassium hydroxide (1.12 g, 20 mmol) was stirred at room temperature under nitrogen for 30 minutes. A solution of trans-3-phenylglycidol (3.0 g, 20 mmol) in dimethylsulfoxide (1 mL) was then added and the mixture was stirred at 70°C for 2 hours until no epoxide remained. The mixture was then cooled and partitioned between water and dichloromethane. The organic layer was separated, washed several times with water, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The crude product was purified via Biotage chromatography (FlasH40i, silica, 10%, 20%, 30% ethyl acetate / hexanes) to afford 1.92 g (36%) of (2RS, 3RS)-3-indole-1- yl-3-phenyl-propane-1,2-diol. 1 HNMR (DMSO): δ3.27 (m, 2H, CH 2 OH), δ4.45(m, 1H, CHOH), δ4.80(t, 1H, CHOH 2 OH), δ5.20 (d, 1H, CHO...
Embodiment 2
[0257] Example 2: (1RS, 2SR)-1-(2,3-dihydro-4H-1,4-benzoxazin-4-yl)-3-(methylamino)-1-phenylpropane- 2-alcohol dihydrochloride
[0258]
[0259] A mixture of 3,4-dihydro-2H-benzo[1,4]oxazine (2.027g, 15.00mmol) and trans-ethyl-3-phenyl glycidate (2.883g, 15.00mmol) was Stir at 135°C for 12 hours. After cooling, the viscous liquid was purified via Biotage Horizon (FLASH 40M, silica, 10%, 20%, 30% EtOAc / hexane) and recrystallized (slightly warmed chloroform / hexane / -20°C) to give 4.261 g (87%) ethyl (2RS, 3RS)-3-(2,3-dihydro-4H-1,4-benzoxazin-4-yl)-2-hydroxy-3-phenylpropionate , as a white solid. MS (ESI) m / z 328.0 ([M+H]+).
[0260] Ethyl (2RS, 3RS)-3-(2,3-dihydro-4H-1,4-benzoxazin-4-yl)-2-hydroxy-3-phenylpropionate (283mg, 0.864 mmol) and methylamine in ethanol (5 mL, 33%) was stirred at 70° C. in a sealed tube for 5 hours. After cooling, all volatiles were removed under reduced pressure. The resulting yellow solid was purified via Biotage Horizon (FLASH 12S, silica, ...
Embodiment 3
[0262] Example 3: (1S,2R)-1-(3-chlorophenyl)-1-(1H-indol-1-yl)-3-(methylamino)propan-2-ol hydrochloride
[0263]
[0264] Step 1: A suspension of sodium hydride (60% dispersion in mineral oil, 4.0 g, 100 mmol) in THF (600 mL) was added dropwise with diethylethoxycarbonylmethylphosphonate (20 mL, 100 mmol) at 23 °C deal with. After 1 hour, 3-chlorobenzaldehyde (9.3 mL, 82 mmol) was added. After an additional 1 hour, the reaction was quenched with water (20 mL) and concentrated in vacuo to remove tetrahydrofuran. The residue was dissolved in ethyl acetate (300 mL), washed with water (5 x 300 mL) and brine (1 x 300 mL), dried (magnesium sulfate) and concentrated in vacuo to give (2E)-3-(3-chlorobenzene base)-ethyl acrylate (18 g, quantitative) as a clear pale yellow oil. MS (ESI) m / z 210 ([M+H]+).
[0265] Step 2: (2E)-3-(3-Chlorophenyl)-ethyl acrylate (17.6 g, 82 mmol) was dissolved in anhydrous dichloromethane (300 mL), cooled to -78° C. Treat with isobutylaluminum hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com