Preparation method of pH sensitive carboxymethyl chitosan hydrogel
A technology of carboxymethyl chitosan water and carboxymethyl chitosan, which is applied in the field of preparation of pH-sensitive dialdehyde starch cross-linked carboxymethyl chitosan hydrogel, can solve the toxic and side effects of human body, and has no pharmaceutical application value, etc. problem, to achieve good pH sensitivity and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
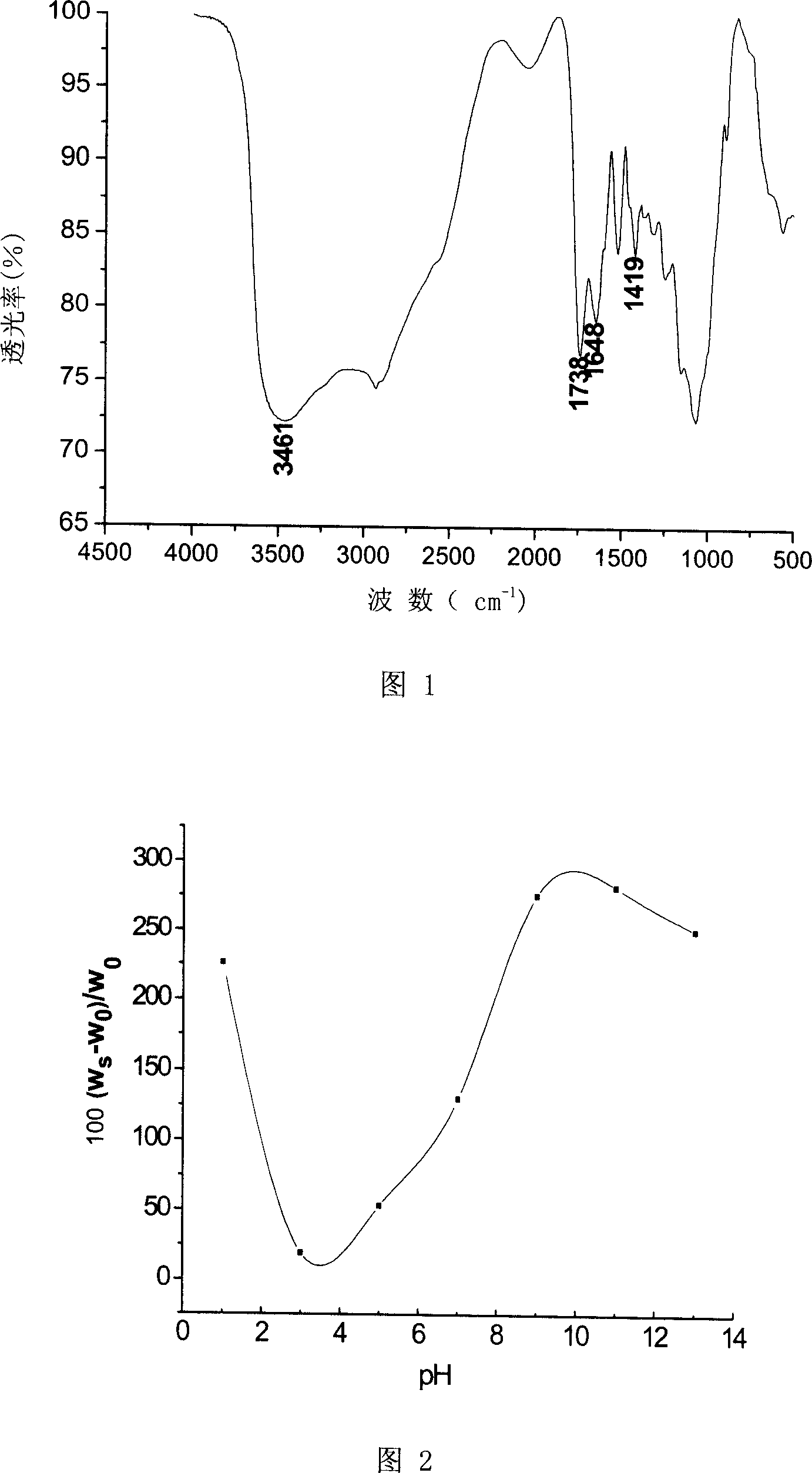
[0018] The carboxymethyl substitution degree is 0.6, the viscosity average molecular weight is 3.5×10 5 Add 5.0 grams of carboxymethyl chitosan into 125 milliliters of distilled water, stir at 70° C. until all dissolve to obtain 4% (weight ratio) clear carboxymethyl chitosan aqueous solution; the degree of oxidation is 60% dialdehyde Add 10.0 grams of starch into 200 milliliters (i.e. 200 grams) of water, stir at 60° C. to make it dissolve as much as possible, and filter to obtain a 5% (by weight) dialdehyde starch aqueous solution. The above carboxymethyl chitosan aqueous solution and dialdehyde starch aqueous solution were stirred and mixed at 50°C for 5 hours according to the weight ratio of 1:0.5. After vacuum defoaming, they were transferred to a test tube and left at room temperature for 15 hours to form a gel. Then the prepared hydrogel is taken out from the test tube, fully washed with deionized water, put into a vacuum oven and dried to constant weight to obtain pH-se...
Embodiment 2
[0021] The carboxymethyl substitution degree is 0.9, the viscosity average molecular weight is 4.5×10 5 3.0 grams of carboxymethyl chitosan joins in 100 milliliters of water, stirs at 60 ℃ until dissolving completely, obtains the clarification carboxymethyl chitosan aqueous solution of 3% (weight ratio); Dialdehyde starch that oxidation degree is 66.8% 10.0 grams were added into 250 milliliters of water, stirred at 50°C to make it dissolve as much as possible, filtered to obtain 4% (by weight) dialdehyde starch aqueous solution. The above-mentioned carboxymethyl chitosan aqueous solution and dialdehyde starch aqueous solution are fully stirred and mixed evenly at 30° C. according to the ratio of 1: 1.5 (weight ratio), transferred to a test tube, vacuum defoamed, and left to stand at room temperature for 18 hours; then The prepared hydrogel was taken out from the test tube, fully washed with deionized water, and vacuum-dried to constant weight to obtain a pH-sensitive carboxyme...
Embodiment 3
[0024] The carboxymethyl substitution degree is 1.2, the viscosity average molecular weight is 5.0×10 5 1.0 gram of carboxymethyl chitosan joins in 100 milliliters of water, stirs at 40 ℃ until dissolving completely, obtains the clarification carboxymethyl chitosan aqueous solution of 1% (weight ratio); Dialdehyde starch that oxidation degree is 50% 15.0 grams were added into 250 milliliters of water, stirred at 80°C to make it dissolve as much as possible, filtered to obtain 6% (by weight) dialdehyde starch aqueous solution. The above-mentioned carboxymethyl chitosan aqueous solution and dialdehyde starch aqueous solution are fully stirred and mixed evenly at 60° C. according to the ratio of 1: 3.0 (weight ratio), transferred to a test tube, vacuum defoamed, and left standing at room temperature for 24 hours; then The prepared hydrogel was taken out from the test tube, fully washed with deionized water to remove impurities, and vacuum-dried to constant weight to obtain pH-sen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com