Transgenic models of alzheimer's disease and uses therefor in the treatment of a variety of neurodegenerative diseases
A technology of transgenic animals and residues, applied in gene therapy, nervous system diseases, disease diagnosis, etc., can solve the problem of unclear exact mechanism of amyloid toxicity, and achieve the goal of preventing the loss of hippocampal synapses and the atrophy of the dentate gyrus Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
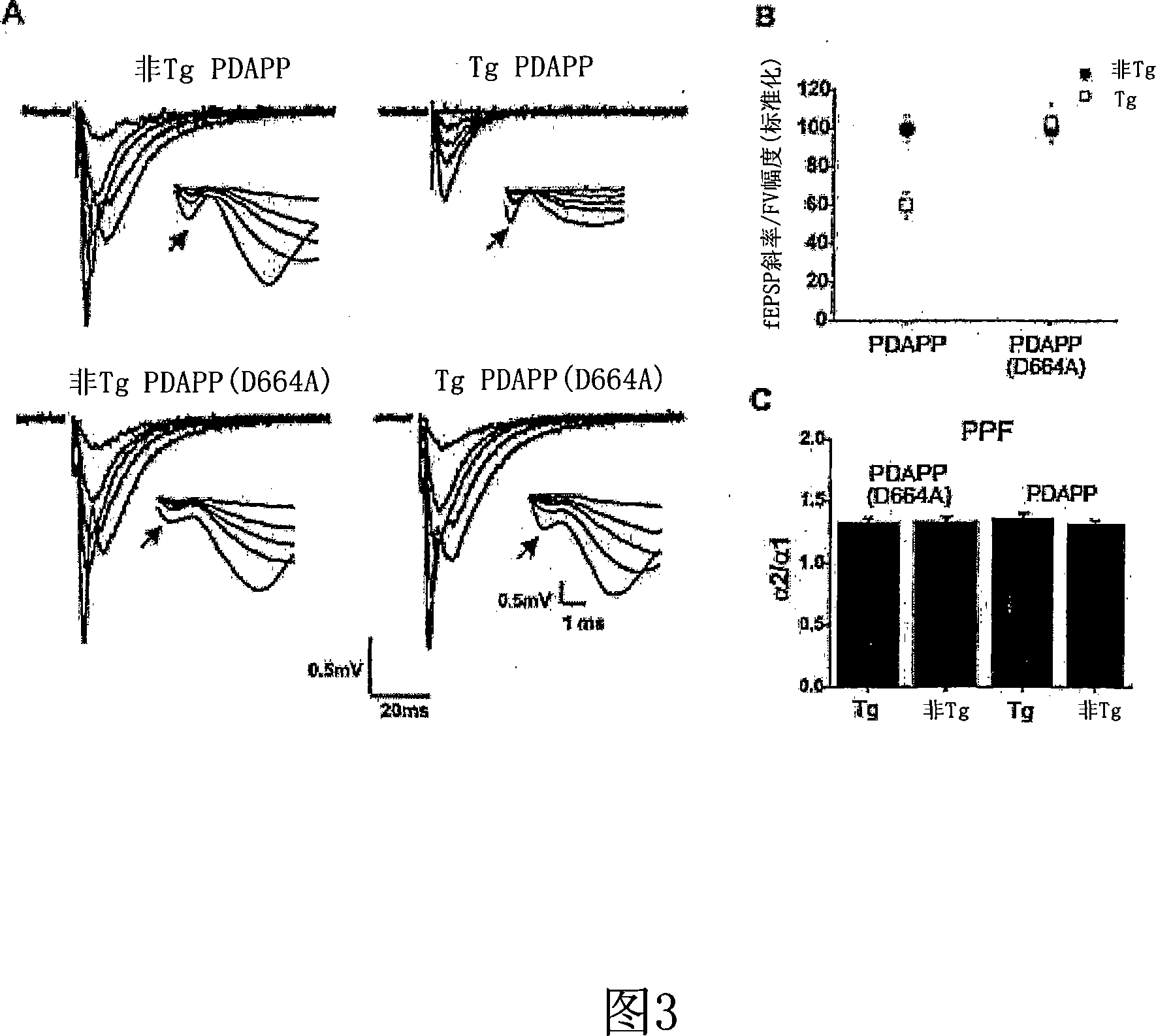
Generation of PDAPP(D664A) Mutation
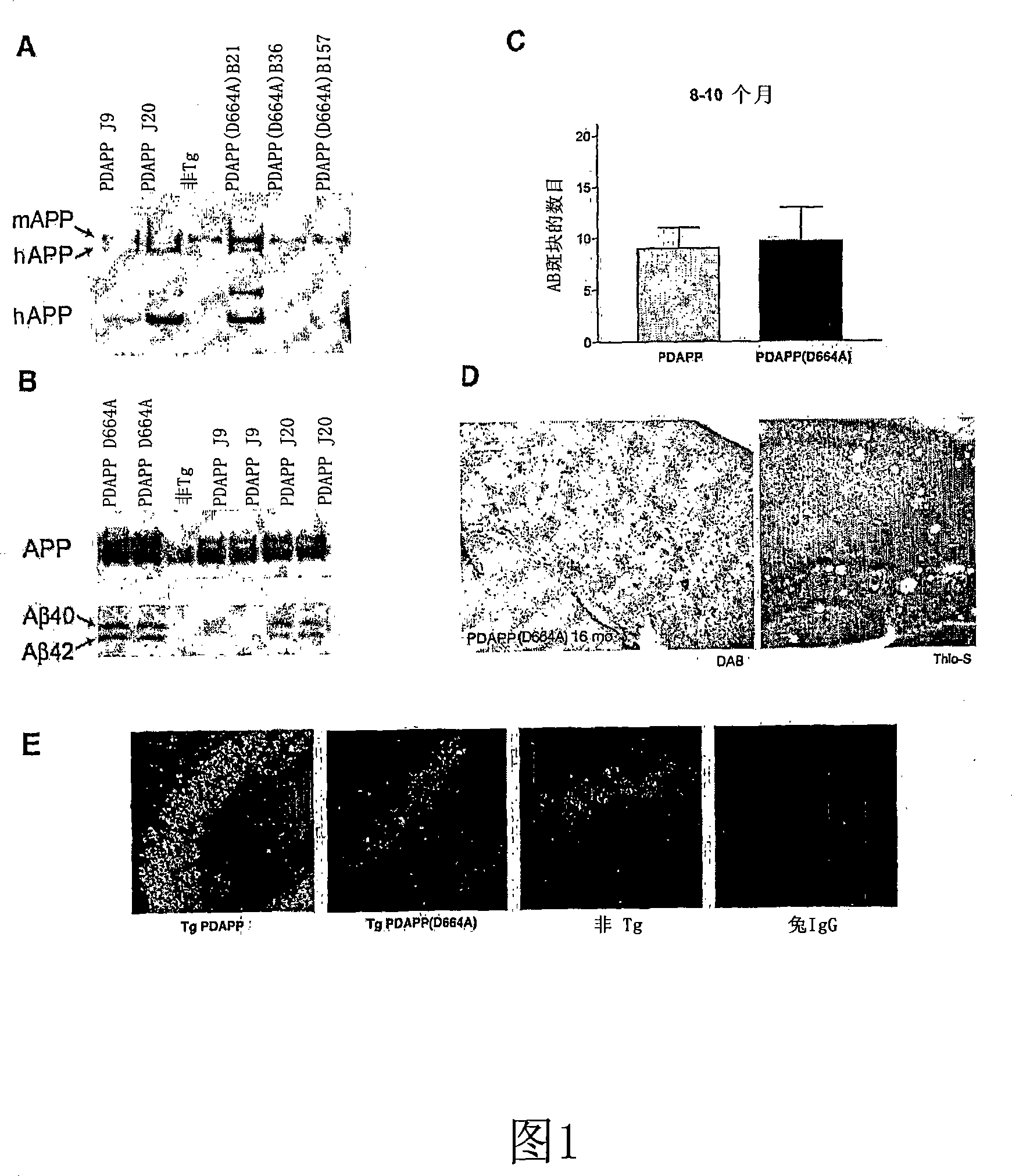
[0109] Point mutations from G to C were introduced into the PDGF β chain promoter-driven hAPP minigene with Swedish and Indiana mutations (see Hsia et al, supra), which mutated Asp664 (APP695 numbering) into is Ala (PDAPP (D664A)). The mutation was confirmed by sequencing and allele-specific amplification.
Embodiment 2
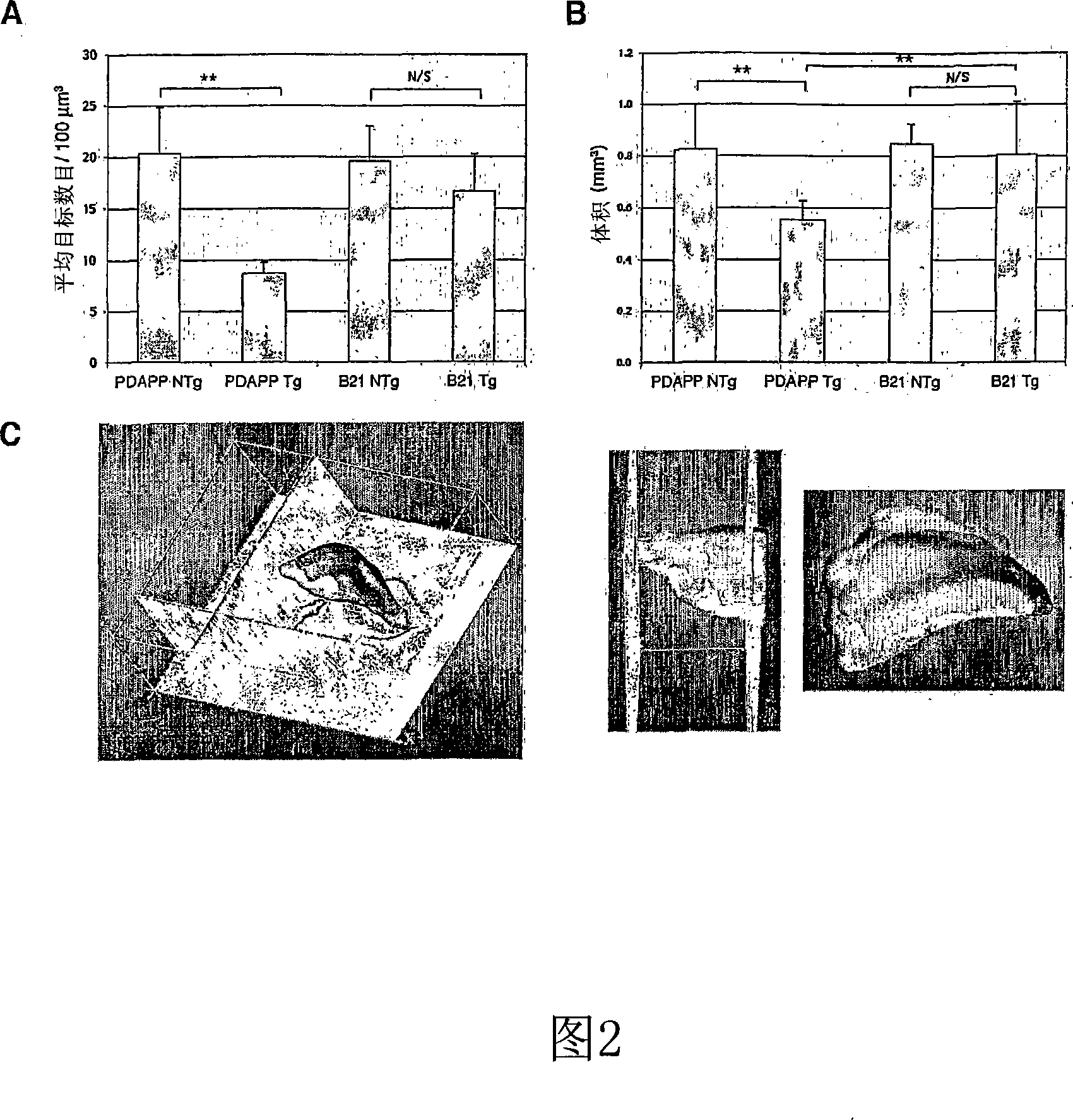
Generation of transgenic mice
[0110] Microinjection of the PDAPP(D664A) transgene was performed as previously described, transgenic founders were identified by PCR, and the PDAPP(D664) B21 strain was selected for this study (see Li, Y., Carlson, E., Murakami, K., Copin, J.C., Luche, R., Chen, S.F., Epstein, CJ. and Chan, P.H., J Neurosci Methods 89, 49-55 (1999)), basically as follows.
[0111] A solution of 2 ng / μl linearized, carrier-sequence-free purified human PDAPP (D664A) transgenic DNA was microinjected into male pronuclei of 1-day-old B6D2F1 / J eggs and transferred into pseudopregnant CD1 surrogates 24 hours later In the womb. Founder identification was performed by PCR using primers specific for the hAPP transgene:
GGTGAGTTTGTAAGTGATGCC (SEQ ID NO: 1), and
TCTTCTTCTTCCACCTCAGC (SEQ ID NO: 2),
and primers specific for the mouse PKR gene:
CAGGCCACTGGGAGGAAAAATG (SEQ ID NO: 3), and
ACCTTCTGTCATGTGGAGGTCC (SEQ ID NO: 4).
[0112] Other transgenic lines prepare...
Embodiment 3
Detection of soluble Aβ
[0114] Aβ levels in the brain were determined from CHAPS soluble lysates using the 26D6 anti-APP monoclonal antibody recognizing residues 1–12 of the Aβ peptide by immunoprecipitation followed by western blot. The immunoprecipitates were fractionated on N-bicine-urea SDS-PAGE gels to separate the A[beta]40 and A[beta]42 species (see Weggen, et al, Nature 414:212-6 (2001)). APP was obtained from immunoblotting of CHAPS brain lysates using CT15, a polyclonal antibody that recognizes the C-terminus of APP. All animals were age-matched between 3-4 months of age prior to deposition of aggregated Aβ peptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com