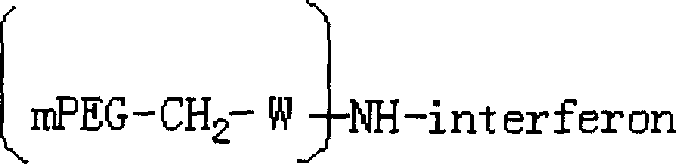
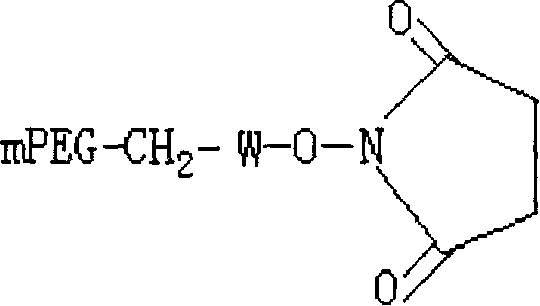
Polyethylene glycol-interferon coupler and its preparation method
A technology of polyethylene glycol and interferon, applied in the direction of interferon, cytokines/lymphokines/interferon, chemical instruments and methods, etc., can solve the problems of increasing interferon, half-life extension is not obvious, and it is broken. Achieve high antiviral activity and long retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1. Preparation of polyethylene glycol-interferon alpha-2a
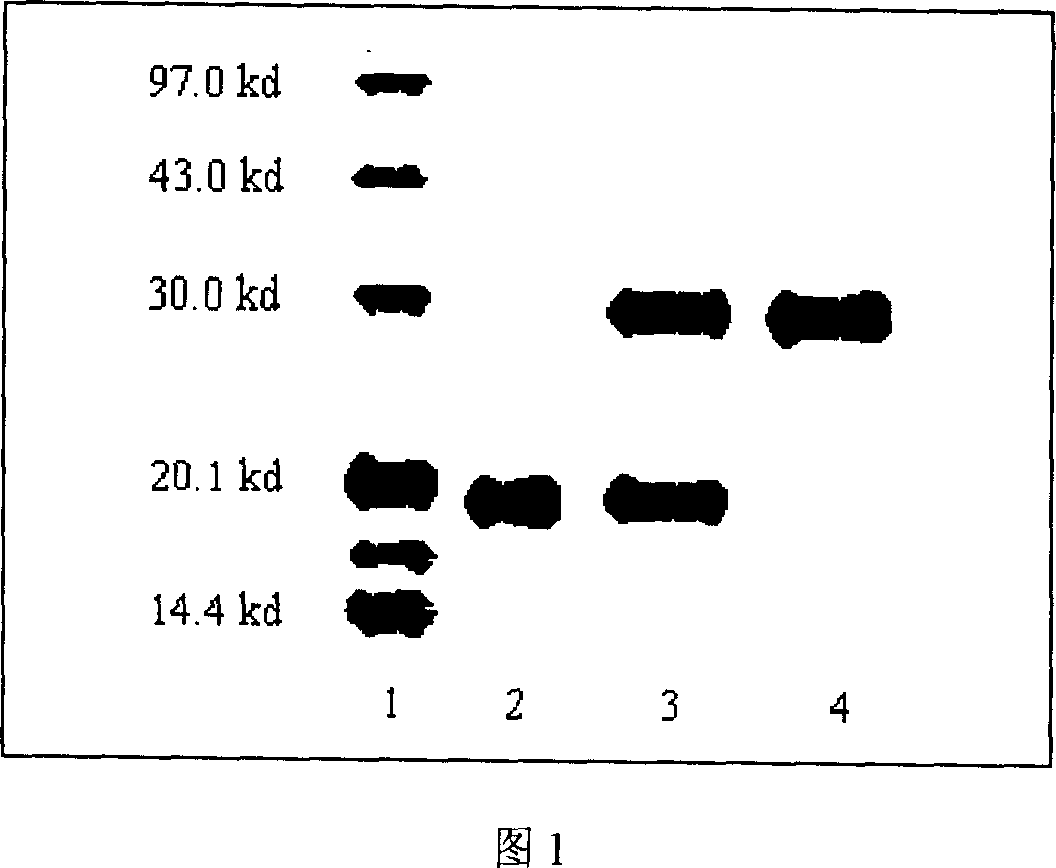
[0024] Dissolve interferon α-2a with 5mM sodium acetate buffer solution pH4.0, and prepare a solution of 8mg / ml; add mPEG-NHS (Mw=10000) according to the ratio of interferon: PEG is 1: 10 , adjust the pH to 85 with sodium hydroxide, react at 25°C for 5 hours, add 0.5ml of 0.5M glycine to terminate the reaction; after 5 minutes, dilute the reaction solution with 6 times the volume of 20mM pH4.0 sodium acetate buffer; The diluted reaction solution was applied to the (Waterman CM-52) column, washed with 6 times the volume of sodium acetate buffer at pH 4.0, and then eluted with sodium acetate buffer containing 05M sodium chloride to collect polyethylene glycol-containing The eluate of alcohol-interferon α-2a was detected by SDS-PAGE electrophoresis. The polyethylene glycol-interferon alpha-2a was further purified with Superdex200, and the eluent was sodium phosphate buffer (containing 0.15M NaCl) at pH...
Embodiment 2
[0025] Embodiment 2. Preparation of polyethylene glycol-composite interferon
[0026] Composite interferon was dissolved in sodium acetate buffer solution of 5mM pH4.0, and was formulated into a solution of 1 mg / ml; mPEG-NHS (Mw=5000, purchased from Sigama company, the same below), adjust the pH to 8.0 with sodium hydroxide, react at 4°C for 24 hours, add 0.2ml of 0.5M glycine to terminate the reaction; after 5 minutes, buffer with 10 times the volume of 20mM sodium acetate pH4.0 Dilute the reaction solution with liquid; apply the diluted reaction solution to a carboxymethyl cellulose column (Waterman CM-52), wash the column with 6 times the volume of sodium acetate buffer at pH 4.0, and wash the column with 0.4M sodium chloride Elute with sodium acetate buffer, collect the eluate containing polyethylene glycol-composite interferon, and detect it by SDS-PAGE electrophoresis. The polyethylene glycol-composite interferon was further purified with Superdex 200, and the eluent wa...
Embodiment 3
[0027] Embodiment 3. Preparation of polyethylene glycol-interferon alpha-2b
[0028] Dissolve interferon α-2b with 5mM sodium acetate buffer solution pH4.5, and prepare a solution of 3 mg / ml; add mPEG-NHS (Mw=20000) at a ratio of 1:15 for interferon:PEG , adjust the pH to 7.5 with sodium hydroxide, react at 25°C for 12 hours, add 0.5ml of 0.5M glycine to terminate the reaction; after 5 minutes, dilute the reaction solution with 5 times the volume of 20mM pH4.0 sodium acetate buffer; The diluted reaction solution was applied to a carboxymethylcellulose column (Waterman CM-52), washed with 6 times the volume of sodium acetate buffer at pH 4.5, and then eluted with sodium acetate buffer containing 0.35M sodium chloride , collect the eluate containing polyethylene glycol-interferon α-2b, and detect it by SDS-PAGE electrophoresis. The polyethylene glycol-interferon α-2b was further purified with Superdex 200, and the eluent was sodium phosphate buffer (containing 0.15M NaCl) at pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com