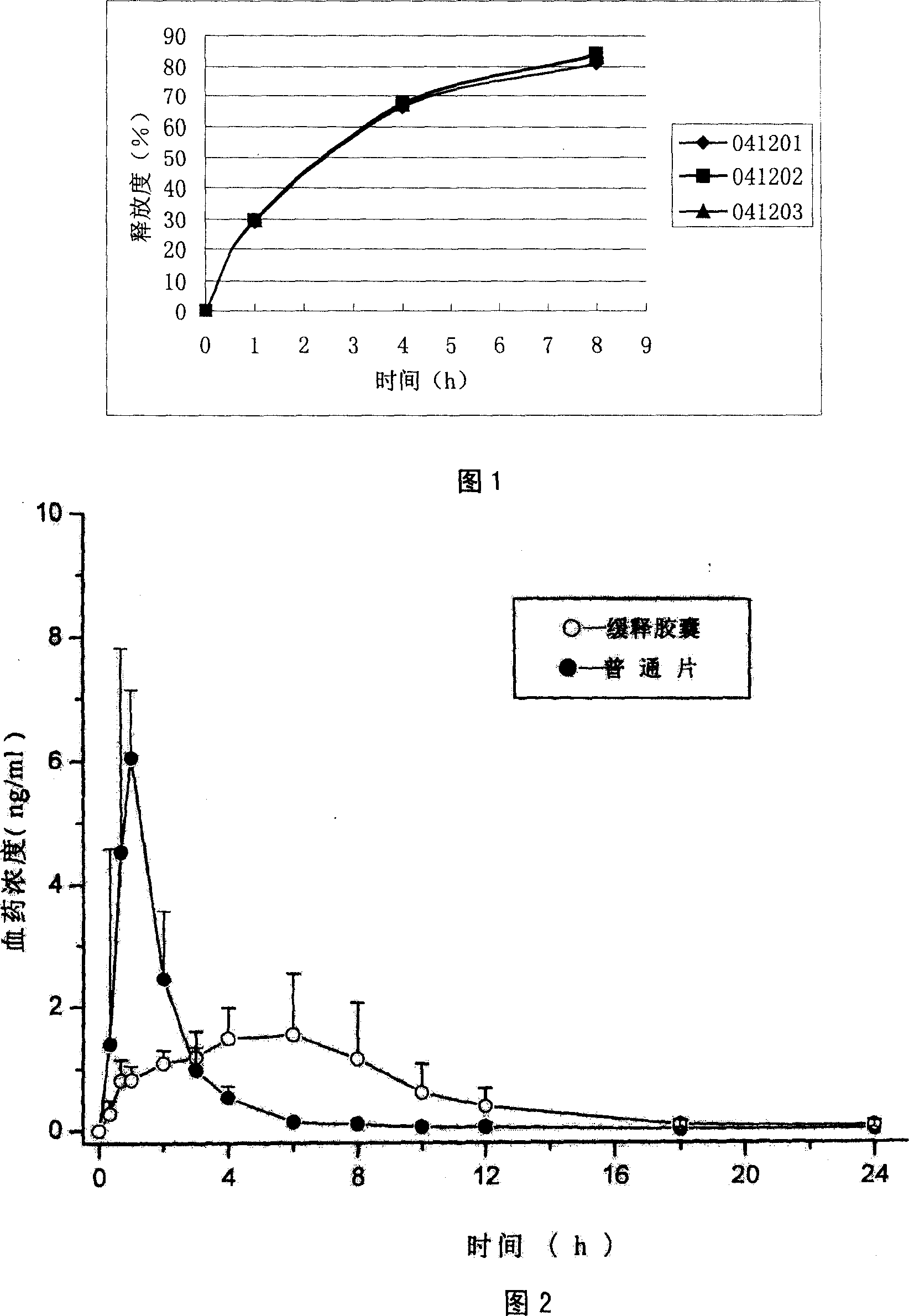
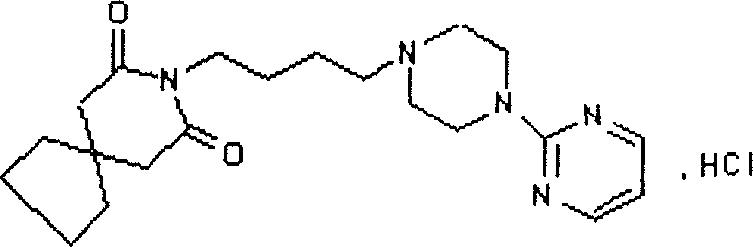
Buspirone hydrochloride slow/controlled release micro pill
A technology of buspirone hydrochloride and pellets, which is applied in the direction of block delivery, organic active ingredients, nervous system diseases, etc., can solve the problems of difficulty in the development of slow/controlled release preparations, and achieve the effect of good slow release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Buspirone Hydrochloride 60g
[0037] Blank ball core 500g
[0038] Hypromellose (6mPa.s) 20g
[0039] Polyethylene glycol 4000 (PEG4000) 4g
[0041] Ethyl cellulose aqueous dispersion 200g
[0042] Appropriate amount of water
[0043] The specific coating process is as follows:
[0044] 1) Weigh 700 / 900 500g of blank pellet core, put it in the fluidized bed, set the equipment parameters, control the inlet air temperature at 61°C, the material temperature at 50°C, and the air volume at 120m 3 / h, the spray pressure is 2×10 5 Pa, using coating solution 1 * Coat the blank core.
[0045] 2) After coating the upper drug layer, take 10% HPMC+0.5% PEG4000 100ml to coat the isolation layer.
[0046] 3) After coating, take the coating solution 2 * Coating of sustained-release coating is carried out.
[0047] 4) Fill the empty capsules according to the specifications (eg, 15 mg or 30 mg per capsule).
[0048] * Coating Solution 1: Weigh 10g of...
Embodiment 2
[0051] Buspirone Hydrochloride 60g
[0052] Blank ball core 500g
[0053] Hypromellose (6mPa.s) 20g
[0054] Polyethylene glycol 4000 (PEG4000) 4g
[0056] Ethyl cellulose aqueous dispersion 200g
[0057] Appropriate amount of water
[0058] 1) Weigh 700 / 900 500g of blank pellet core, put it in the fluidized bed, set the equipment parameters, control the inlet air temperature at 61°C, the material temperature at 50°C, and the air volume at 120m 3 / h, the spray pressure is 2×10 5 Pa, using coating solution 1 * Coat the blank core.
[0059] 2) After coating the upper drug layer, take 10% HPMC+0.5% PEG4000 100ml to coat the isolation layer.
[0060] 3) After coating, take the coating solution 2 * Coating of sustained-release coating is carried out.
[0061] 4) After wrapping the slow-release coat, cure at a suitable temperature for 24 hours.
[0062] 5) Fill the empty capsules according to the specifications (eg, 15 mg or 30 mg per capsule).
...
Embodiment 3
[0066] Buspirone Hydrochloride 60g
[0067] Blank ball core 500g
[0068] Hypromellose (6mPa.s) 20g
[0069] Polyethylene glycol 4000 (PEG4000) 4g
[0071] Ethylcellulose aqueous dispersion 374g
[0072] Appropriate amount of water
[0073] The specific coating process is as follows:
[0074] 1) Weigh 700 / 900 500g of blank pellet core, put it in the fluidized bed, set the equipment parameters, control the inlet air temperature at 61°C, the material temperature at 50°C, and the air volume at 120m 3 / h, the spray pressure is 2×10 5 Pa, using coating solution 1 * Coat the blank core.
[0075] 2) After coating the upper drug layer, take 10% HPMC+0.5% PEG4000 100ml to coat the isolation layer.
[0076] 3) After coating, use coating solution 2 * Surelease is used for the coating of sustained release coating.
[0077] 4) Fill the prepared sustained-release pellets into capsules containing 15 mg of buspirone hydrochloride, and measure the release cur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com