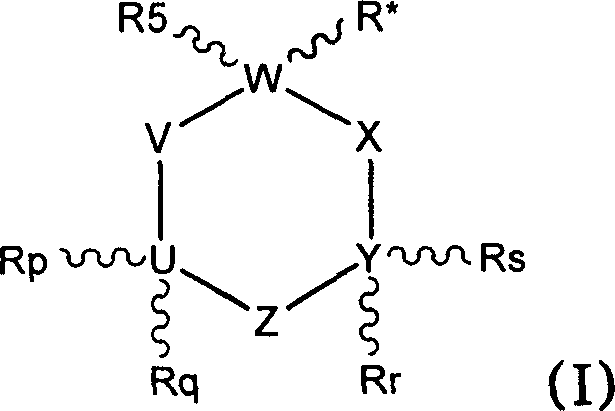
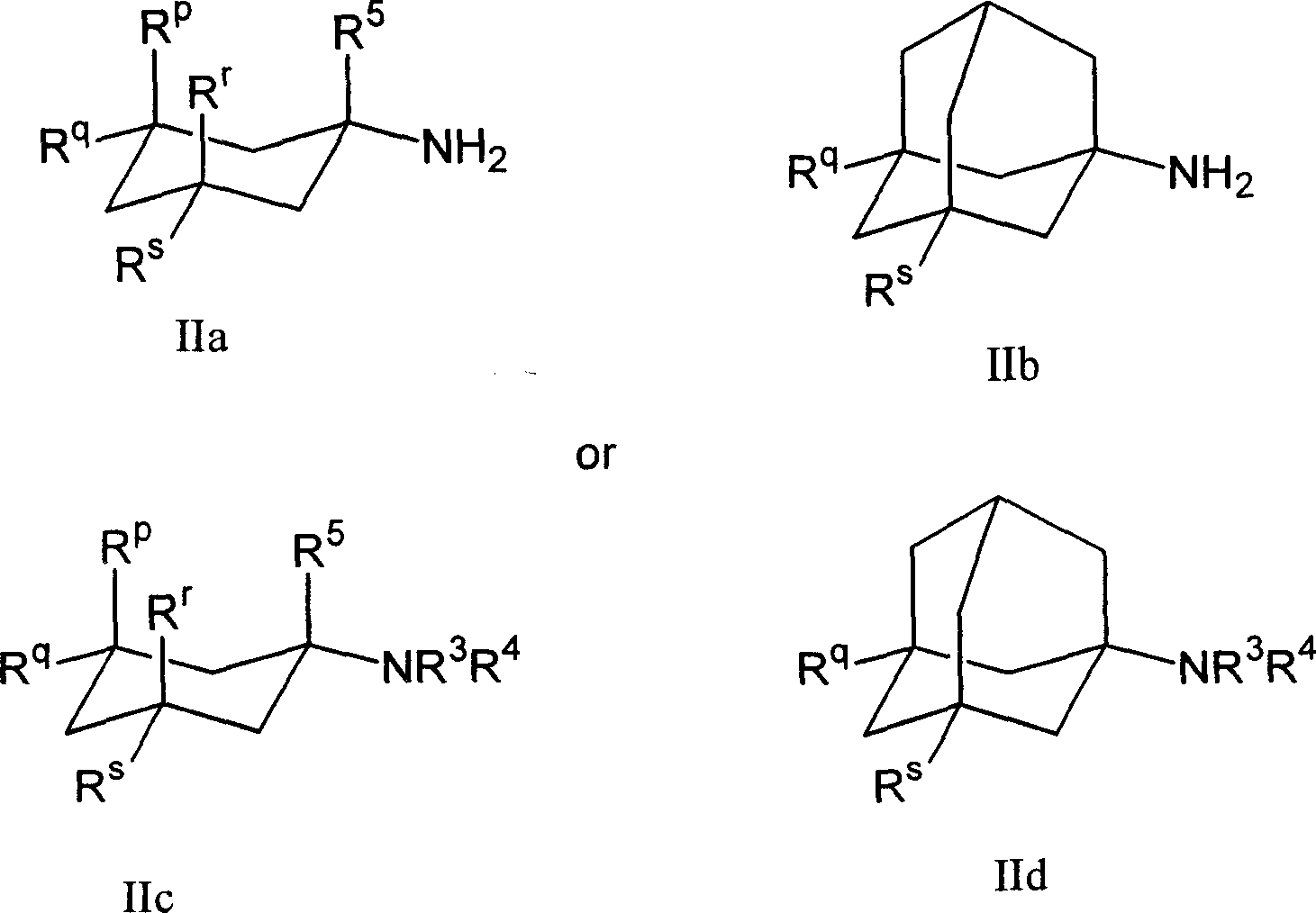
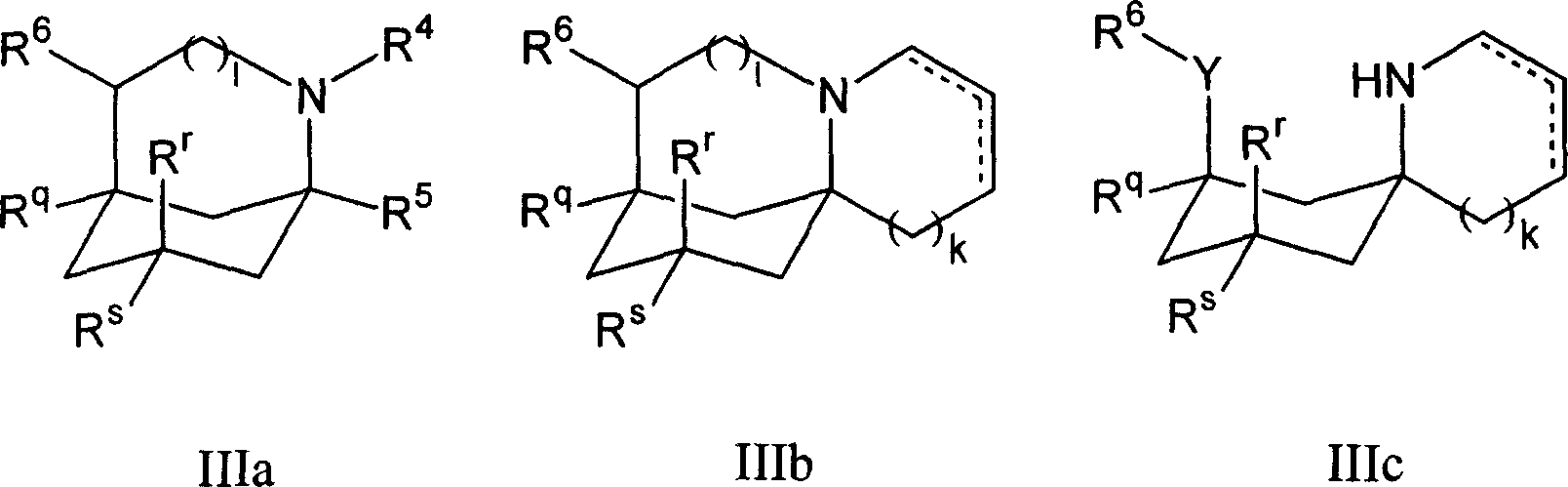
1-aminocyclohexane derivatives for the treatment of agitation and other behavioral disorders, especially those associated with alzheimer's disease
An aminocyclohexane, agitation technology, applied in the field of 1-aminocyclohexane derivatives for the treatment of agitation and other behavioral disorders, especially those diseases related to Alzheimer's disease, can solve the problem of Alternatives to benzodiazepines _ treatment and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0286] Treatment example 1: Memantine in patients with moderate to severe Alzheimer's disease behavioral consequences
[0287] Patients with moderate to severe AD were randomized to either placebo or 20 mg daily memantine for 28 weeks. The first efficacy variables were impressions of change based on clinician's interview plus information provided by caregiver (CIBIC-Plus) and Alzheimer's Disease Collaborative Study Activities of Daily Living Record (ADCS-ADL) altered for severe dementia. Secondary efficacy variables included severe impairment (SIB) and neuropsychiatric records (NPI). Treatment differences between baseline and endpoints were assessed. Incomplete observations take the most recent previous observation (last observation carried forward - LOCF). Results were also analyzed using only observed values where missing values were not replaced (analysis of observed cases (OC)). AD patients (N=252), (67% female, mean age=76 years) were randomized from 32 US clin...
Embodiment 2
[0318] Treatment example 2: In patients with moderate to severe Alzheimer's disease, memantine and Effects of co-administration of donepezil on behavioral outcomes
[0319]A 24-week, double-blind, placebo-controlled trial was conducted in moderate to severe Alzheimer's disease patients (N=404) undergoing donepezil therapy and randomized to memantine or placebo. Behavioral symptoms were assessed using the NPI at baseline, week 12, and the final visit (week 24). The trial has identified benefits of memantine on functional, cognitive and global measures. Statistical analysis was based on the ITT population using the carried forward last observation (LOCF) and observed cases (OC) approach.
[0320] Therapeutic benefit of memantine in combination with stable doses of commonly used AChEIs was observed on behavioral (NPI) measures in patients with moderate to severe Alzheimer's disease (MMSE mean at entry 10). Memantine treatment resulted in significant improvements compared to...
Embodiment 3
[0373] This example presents the results of five (5) clinical trials, two of which are described above in Example 1 (MZ-9065) and Example 2 (MD-02). In addition, the results of two additional trials, MD-01, MD-10 and MD-12 are presented.
[0374] MD-01: MD-01 is a trial evaluating memantine as monotherapy for 24 weeks in patients with moderate to severe AD. Approximately 350 patients participated. Efficacy was assessed using Severe Impairment (SIB), Assessment of Daily Living (ADL) and CIBIC-plus Scales.
[0375] MD-10: MD-10 is a randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with mild to moderate dementia of the Alzheimer's type. The primary endpoints of MD-10 are ADAS-cog and CIBIC-plus. Other endpoints of the MD-10 include ADCS-ADL, NPI and RUD (Resource Utilization in Dementia).
[0376] MD-12 : MD-12 is a randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com