Method for capillary electrophoresis electrochemiluminescence detection of metoprolol and atenolol
A technology of capillary electrophoresis and luminescence detection, which is applied in the direction of chemiluminescence/bioluminescence, electrochemical variables of materials, and analysis by making materials undergo chemical reactions. It can solve the problems of high instrument cost and achieve low cost, high sensitivity and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
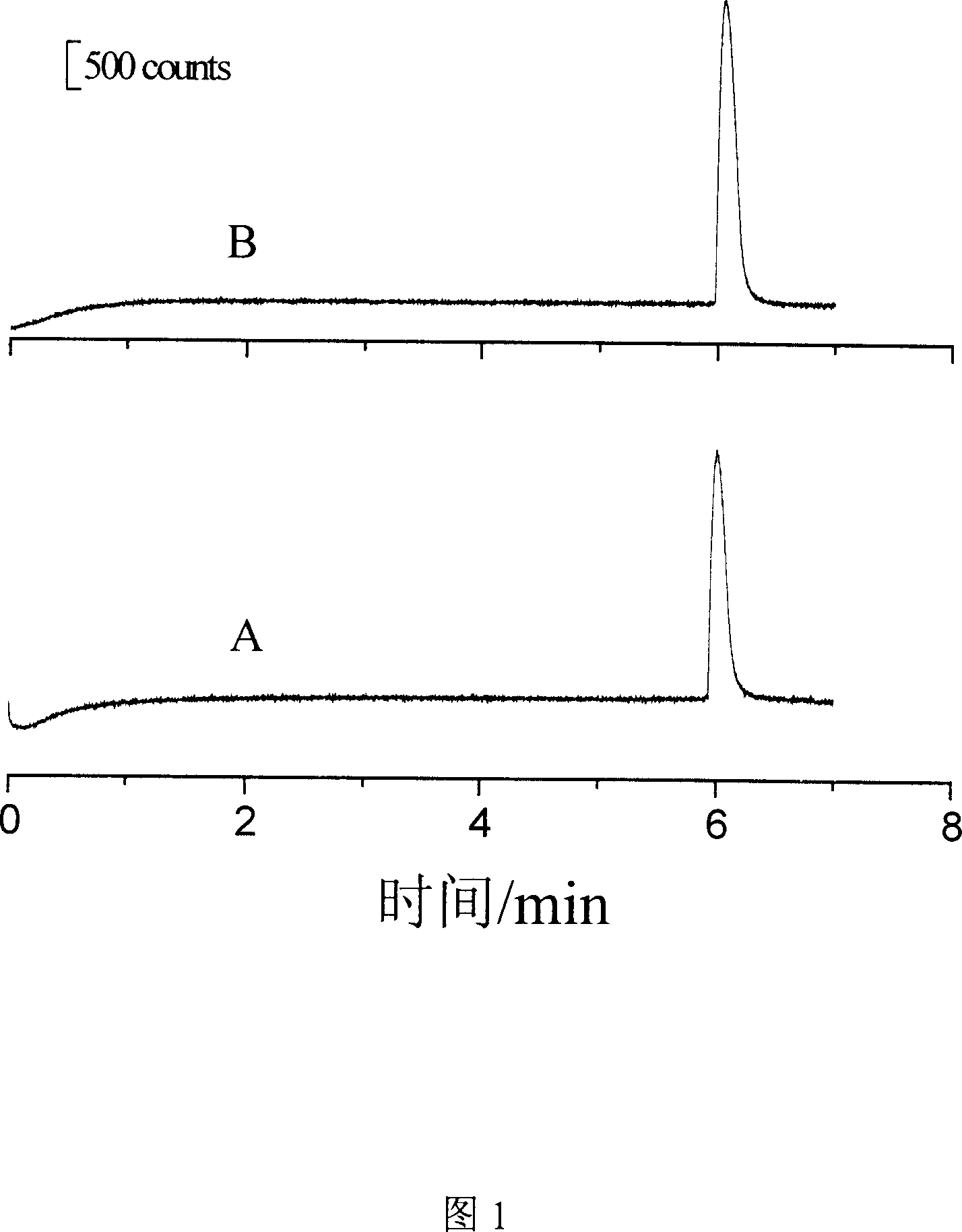
[0041] The detection of embodiment 1 metoprolol and atenolol standard substance
[0042] For the detection of metoprolol and atenolol standard products, the instrument used is as described above, and the detection steps are as follows:
[0043] 1. Preparation of standard solutions of metoprolol and atenolol
[0044] The stock solution concentration of metoprolol and atenolol was 1.0mmol / L. The stock solution was stored in a refrigerator at 4°C, protected from light.
[0045] 2.10.0mmol / L, NaH at pH8.5 2 PO 4 -Na 2 HPO 4 Preparation of buffer solution
[0046] First prepare NaH with a concentration of 10.0mmol / L 2 PO 4 solution and Na at a concentration of 10.0mmol / L 2 HPO 4 solution, the two were then mixed and adjusted to pH 8.5.
[0047] 3.100.0mmol / L, NaH at pH8.5 2 PO 4 -Na 2 HPO 4 Preparation of buffer solution
[0048] First prepare NaH with a concentration of 100.0mmol / L 2 PO 4 solution and a concentration of 100.0mmol / L Na 2 HPO 4 solution, and then...
Embodiment 2
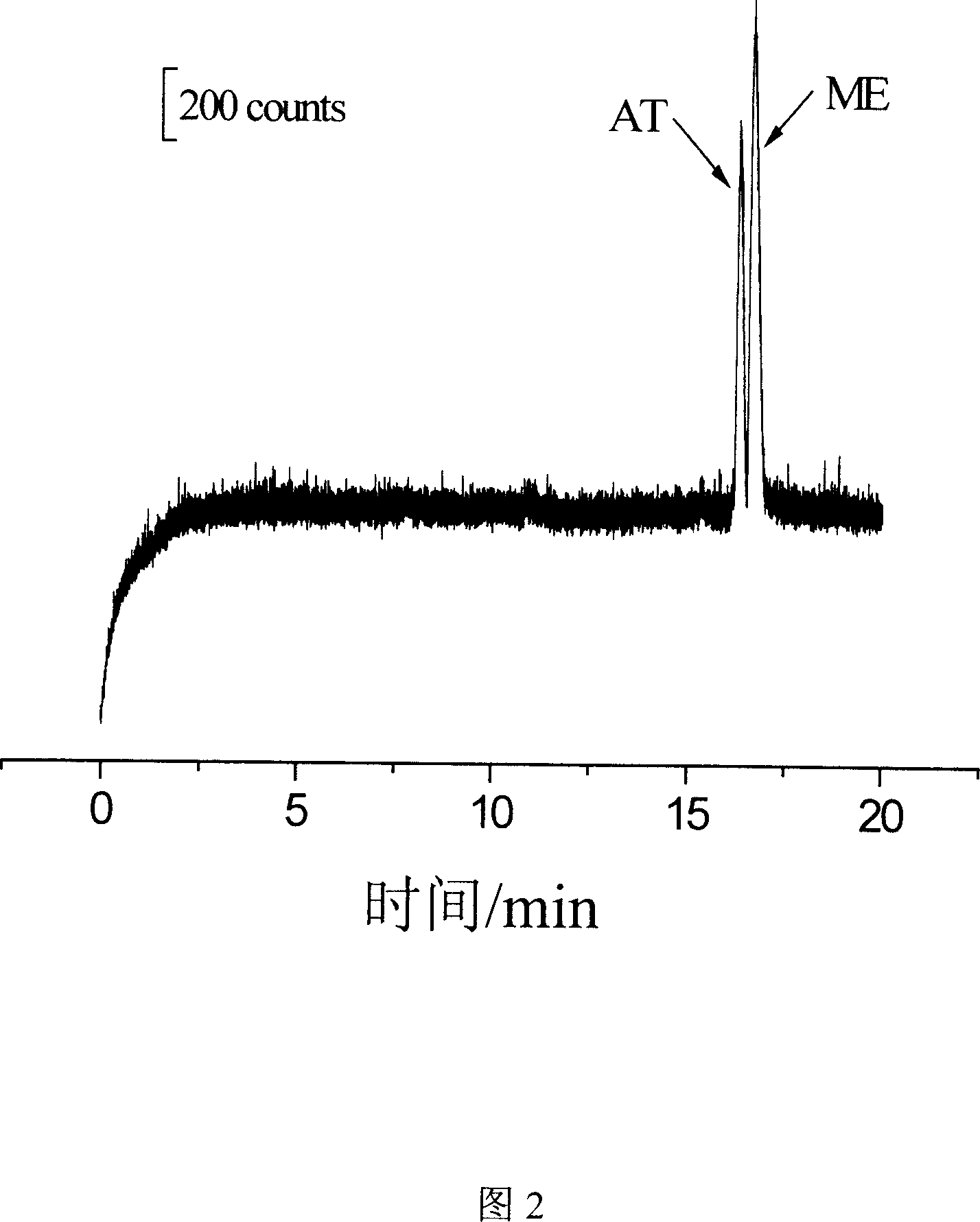
[0054] Example 2 Simultaneous Separation and Detection of Metoprolol and Atenolol Standards
[0055] For the simultaneous separation and detection of metoprolol and atenolol standards, the instruments used are as described above, and the separation and detection steps are as follows:
[0056] 1. Preparation of standard solutions of metoprolol and atenolol
[0057] The stock solution concentration of metoprolol and atenolol was 1.0mmol / L. The stock solution was stored in a refrigerator at 4°C, protected from light.
[0058] 2.10.0mmol / L, NaH at pH3.0 2 PO 4 -Na 2 HPO 4 Preparation of buffer solution
[0059] First prepare NaH with a concentration of 10.0mmol / L 2 PO 4 Solution and H at a concentration of 1.0mmol / L 3 PO 4 solution, followed by H 3 PO 4 The solution was adjusted to pH 3.0.
[0060] 3.100.0mmol / L, NaH at pH8.5 2 PO 4 -Na 2 HPO 4 Preparation of buffer solution
[0061] First prepare NaH with a concentration of 100.0mmol / L 2 PO 4 solution and a co...
Embodiment 3
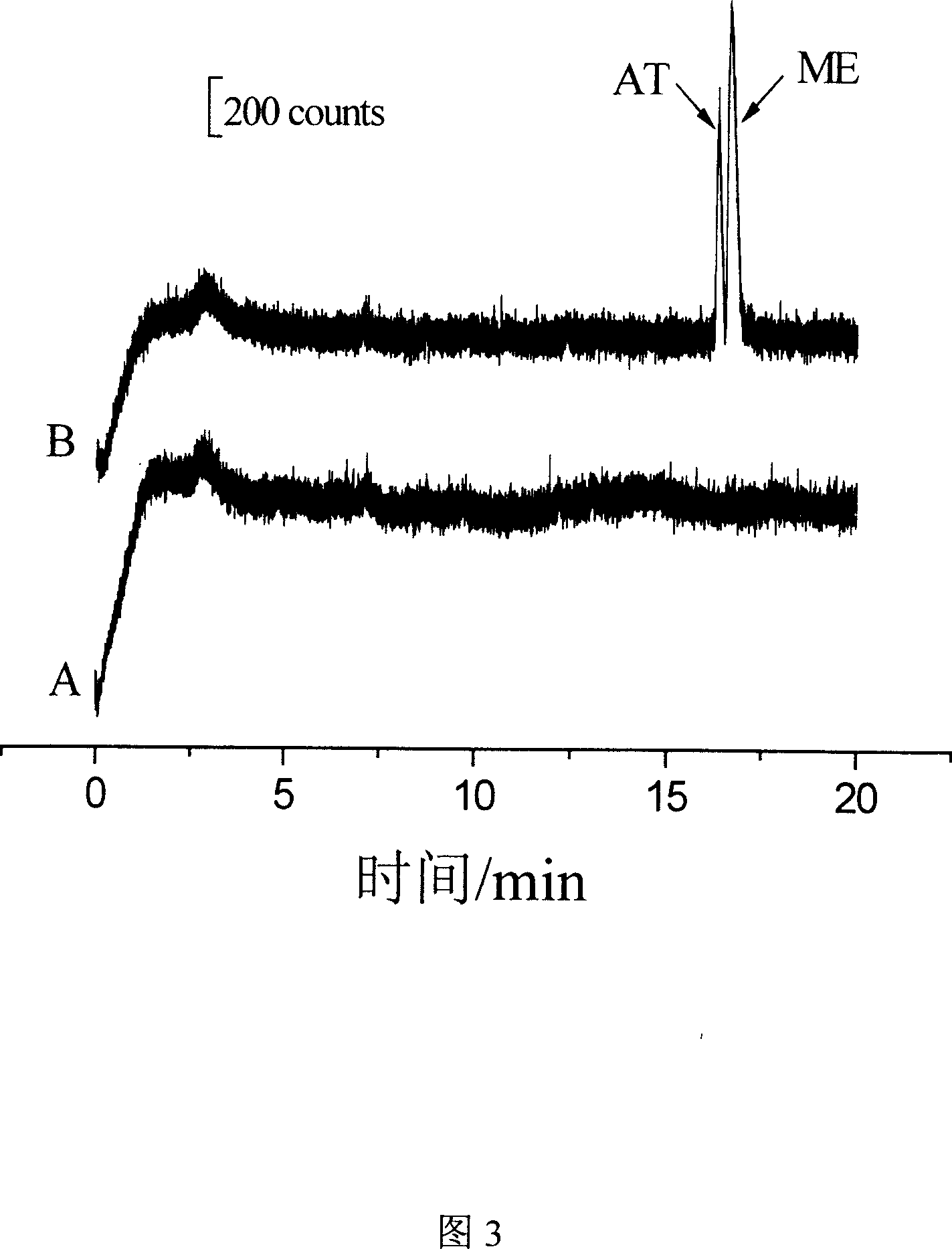
[0067] Example 3 Separation and detection of metoprolol and atenolol in urine samples
[0068] For the separation and detection of metoprolol and atenolol in urine samples, the instruments used are as described above, and the separation and detection steps are as follows:
[0069] 1.10.0mmol / L, NaH at pH3.0 2 PO 4 -Na 2 HPO 4Preparation of buffer solution
[0070] First prepare NaH with a concentration of 10.0mmol / L 2 PO 4 Solution and H at a concentration of 1.0mmol / L 3 PO 4 solution, followed by H 3 PO 4 The solution was adjusted to pH 3.0.
[0071] 2.100.0mmol / L, NaH at pH8.5 2 PO 4 -Na 2 HPO 4 Preparation of buffer solution
[0072] First prepare NaH with a concentration of 100.0mmol / L 2 PO 4 solution and a concentration of 100.0mmol / L Na 2 HPO 4 solution, and then mix the two to adjust the pH to 8.5.
[0073] 3. The preparation process of the blank urine sample and standard urine samples spiked with metoprolol and atenolol is as follows:
[0074] Take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com