Low disturbance pulsatile flow system
a flow system and low disturbance technology, applied in the field of coronary implants, can solve the problems of high flow-dependent nature of thrombosis, major biocompatibility, acute or long-term device failure, etc., and achieve the effect of minimizing background noise, minimizing length and discontinuities, and reducing the effect of thrombotic signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
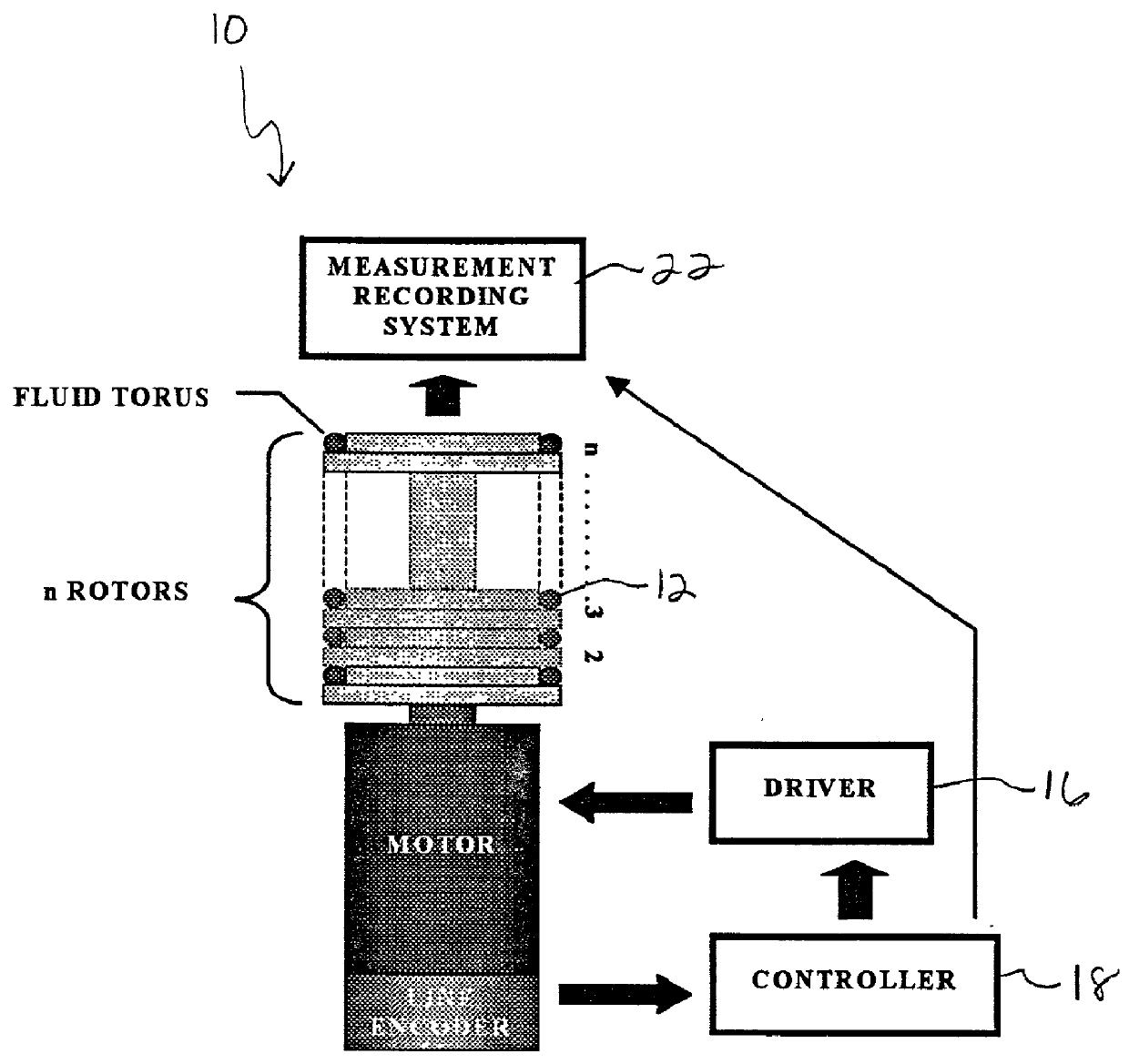
[0034] As shown initially in FIG. 1, is a low-disturbance, pulsatile, in vitro flow device is generally
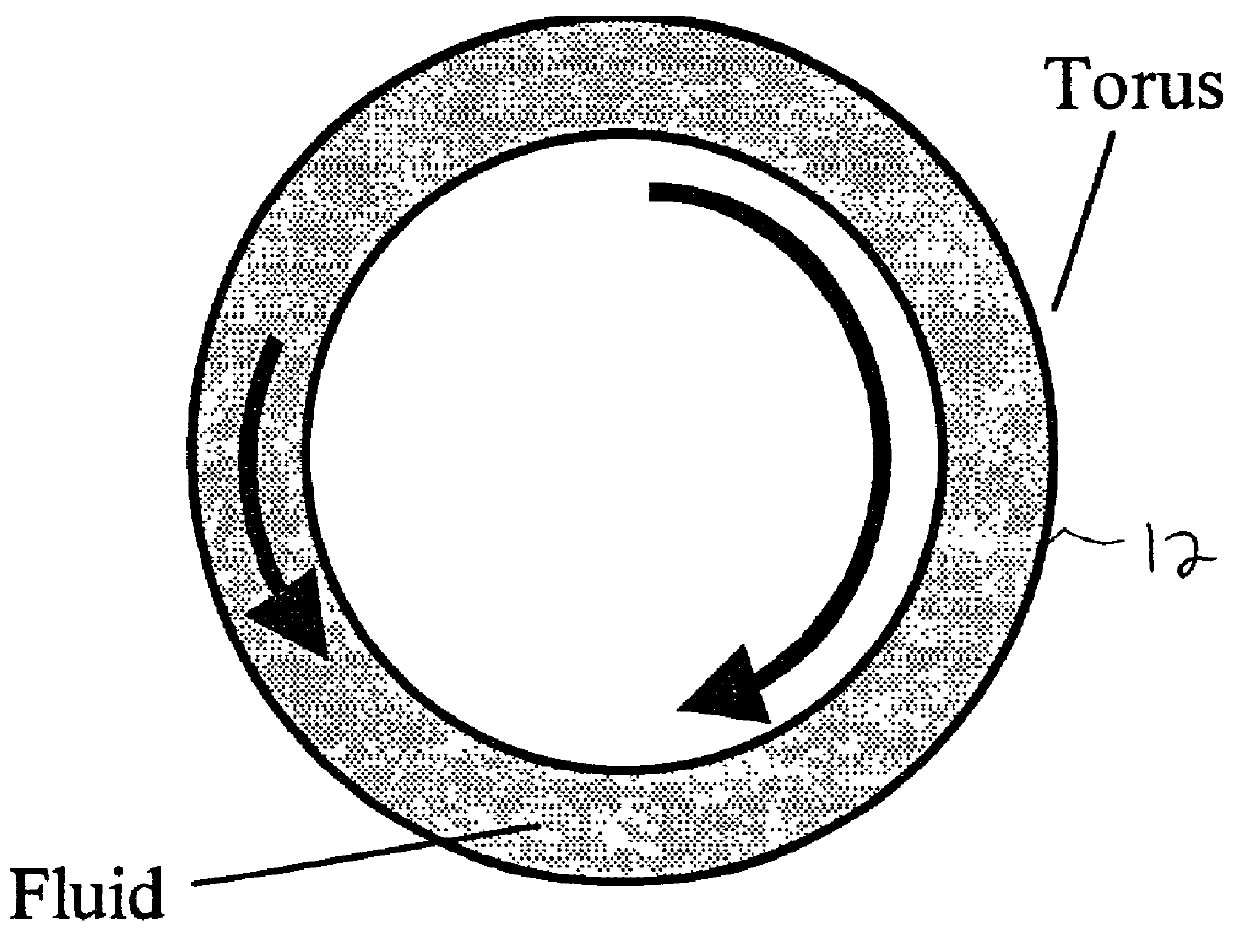
[0035] shown at 10. The device includes a fluid torus 12, rotor-stage 14, driving motor 16, motion controller 16, and a measurement system 20 utilized to observe the physiological, controllable flows in a manner to create a large thrombotic signal. The system is usually utilized in an incubator, not shown, to keep the samples at a stable temperature. As described in detail below, this includes placing a stent 24 or a graft in a torus or loop 12, as seen in the Figures. The loop 12 is then filled with the desired blood constituents and spun about its axis in a prescribed fashion. This spinning is controlled in such a way as to modulate the inertial flow of the contained fluid through transmitted shear forces from the tubing wall, thereby creating a low disturbance flow.
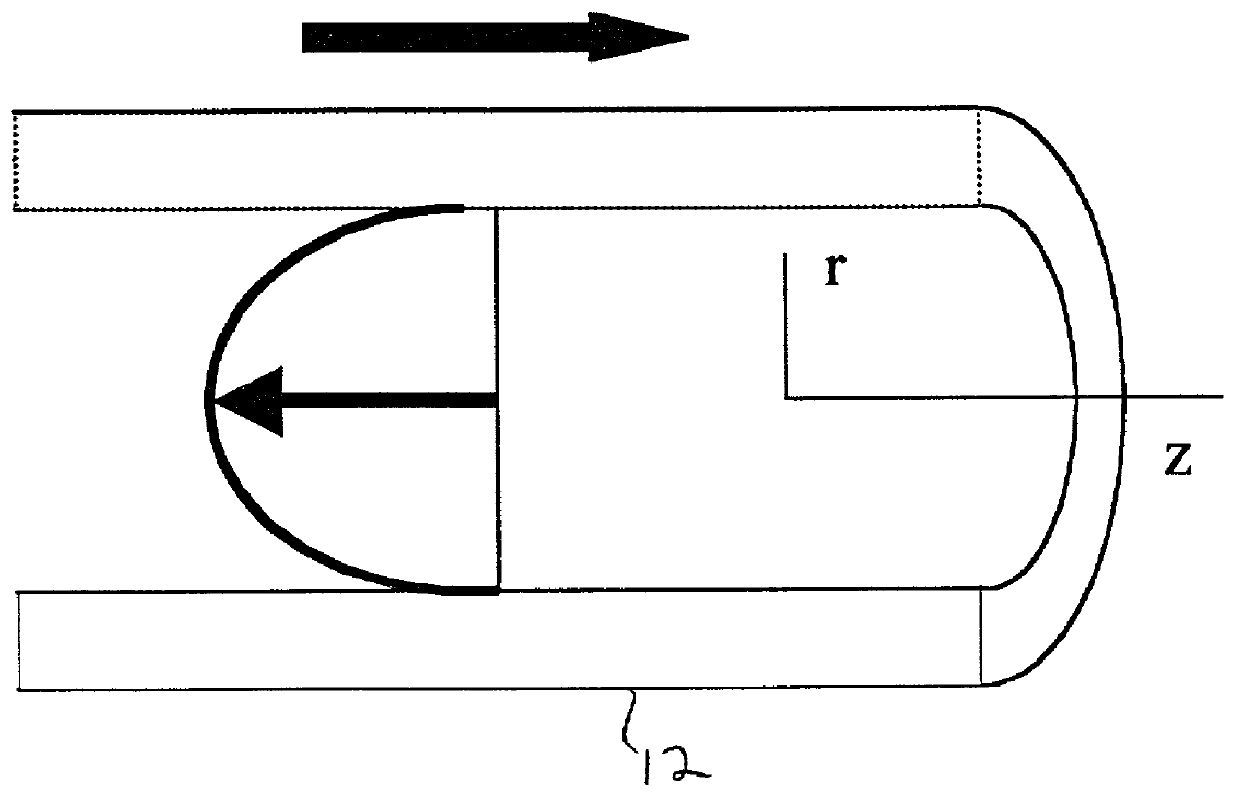
[0036] To create the desired flow profiles, the fluid-filled torus 12 is rotated about its axis. When impulsively s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com