Methods for protein transfer
a protein and protein technology, applied in the field of protein transfer, can solve the problems of difficult expressing more than a costimulator (or coinhibitor), cumbersome and time-consuming transfection process, and often poorly transfectable apcs, including tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
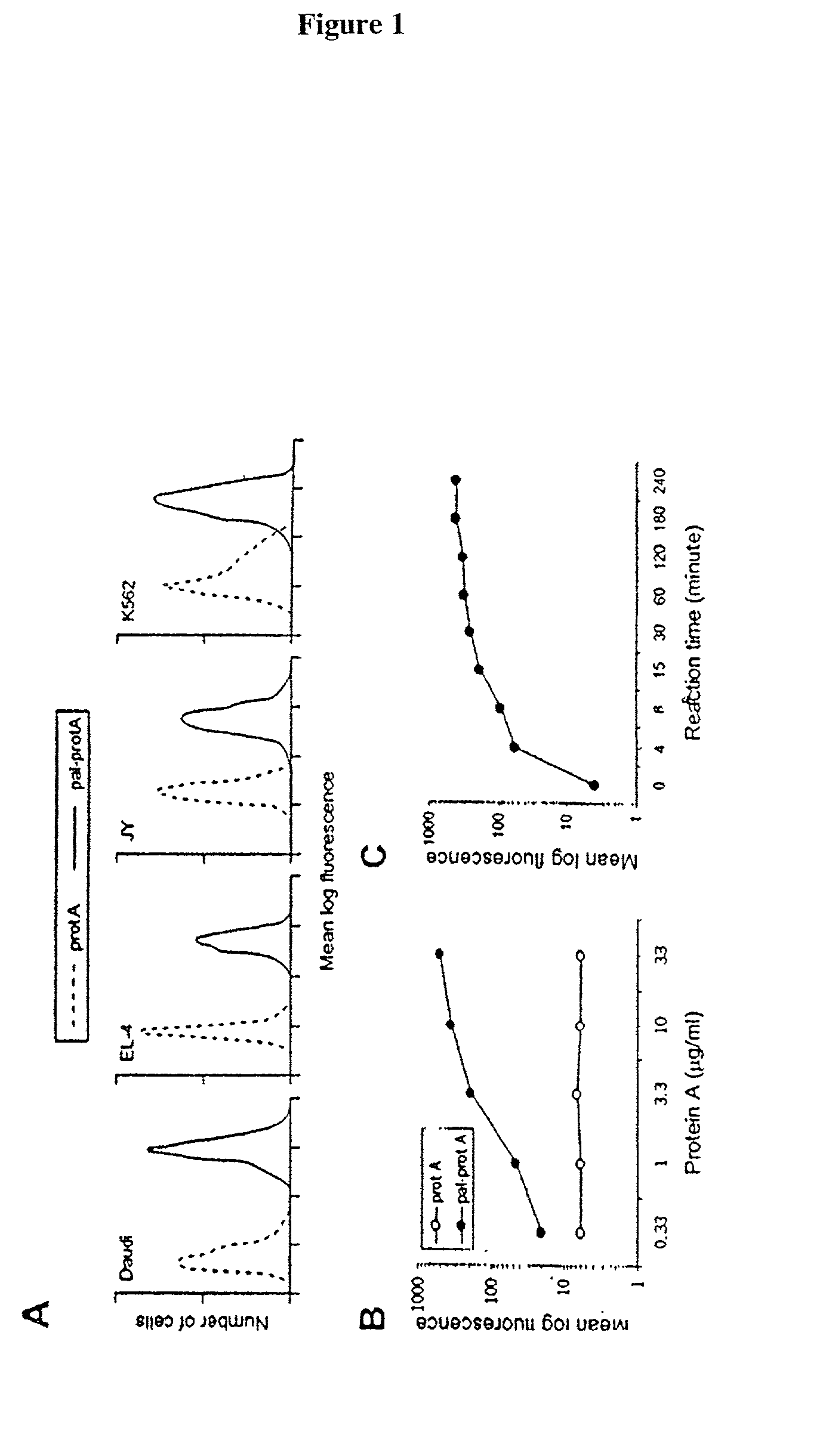
[0042] The following example demonstrates a method for transferring a B7-1-Fc.gamma..sub.1 fusion protein to a cell using palmitated protein A.
[0043] Palmitation of protein A
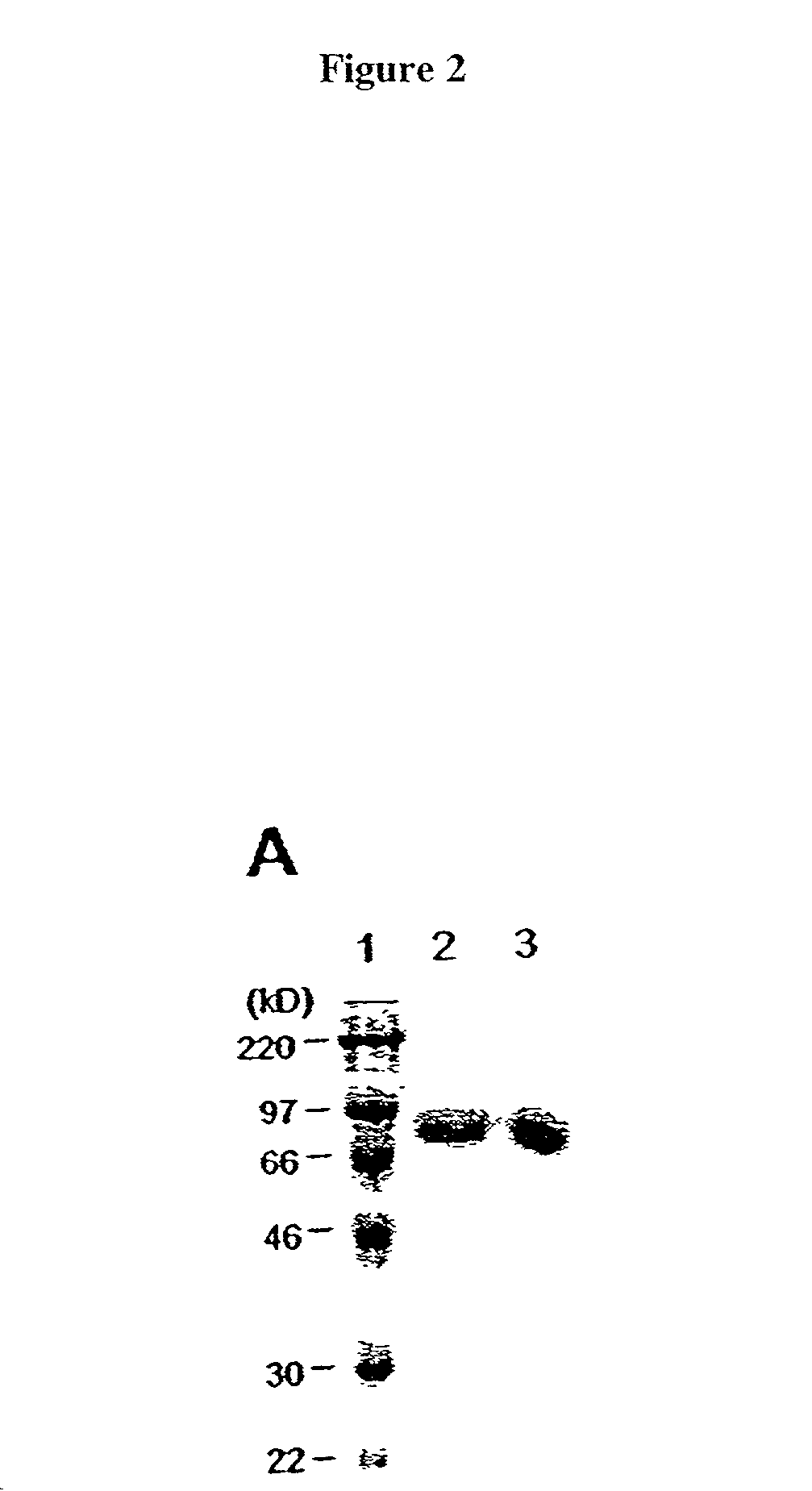
[0044] Recombinant protein A (Calbiochem, La Jolla, Calif.) was derivatized with the N-hydroxysuccinimide ester of palmitic acid (Sigma, St. Louis, Mo.) as described by Kim and Peacock, J. Immunol Methods, 158:57 (1993). Briefly, a stock solution of the N-hydroxysuccinimide ester of palmitic acid was made, as was a solution containing protein A in a concentration of about 1.5 mg / ml. The solutions were mixed in a ratio of about 10 .mu.g ester per ml protein and incubated at room temperature with constant mixing for about 18 h. The lipid-derivatized protein A was purified as described by Huang, et al., J. Biol. Chem., 225:8015 (1980) using a 30-ml Sephadex G-25 (Sigma) column. The protein product, referred to herein as "pal-prot A", was quantitated using a bicinchoninic acid kit (Bio-Rad, Richmond, Calif.), filter...
example 2
[0070] The effect of temperature on membrane-incorporated protein A was studied; the transferred protein must remain cell-bound in vivo in order to prime T-cells, which requires stable engagement of costimulators for at least several hours. It was determined that the reaction temperature at which a lipidated protein is transferred to the cell membrane has a major impact on long-term retention of the protein on the membrane. Protein transfer reactions were performed at 4.degree. C., 25.degree. C. or 37.degree. C.; palmitated protein A was transferred onto K562 cells. An hB7-1.multidot.Fc was .sup.125I-labeled and transferred to the protein A-coated cells in the manner described in Example 1. To prevent interference of endocytosis likely to occur at temperatures above 4.degree. C., the cells were treated with the metabolic inhibitors sodium azide and 2-deoxyglucose prior to the transfer reaction.
[0071] To determine the long-term retention of the transferred protein on the cell membran...
example 3
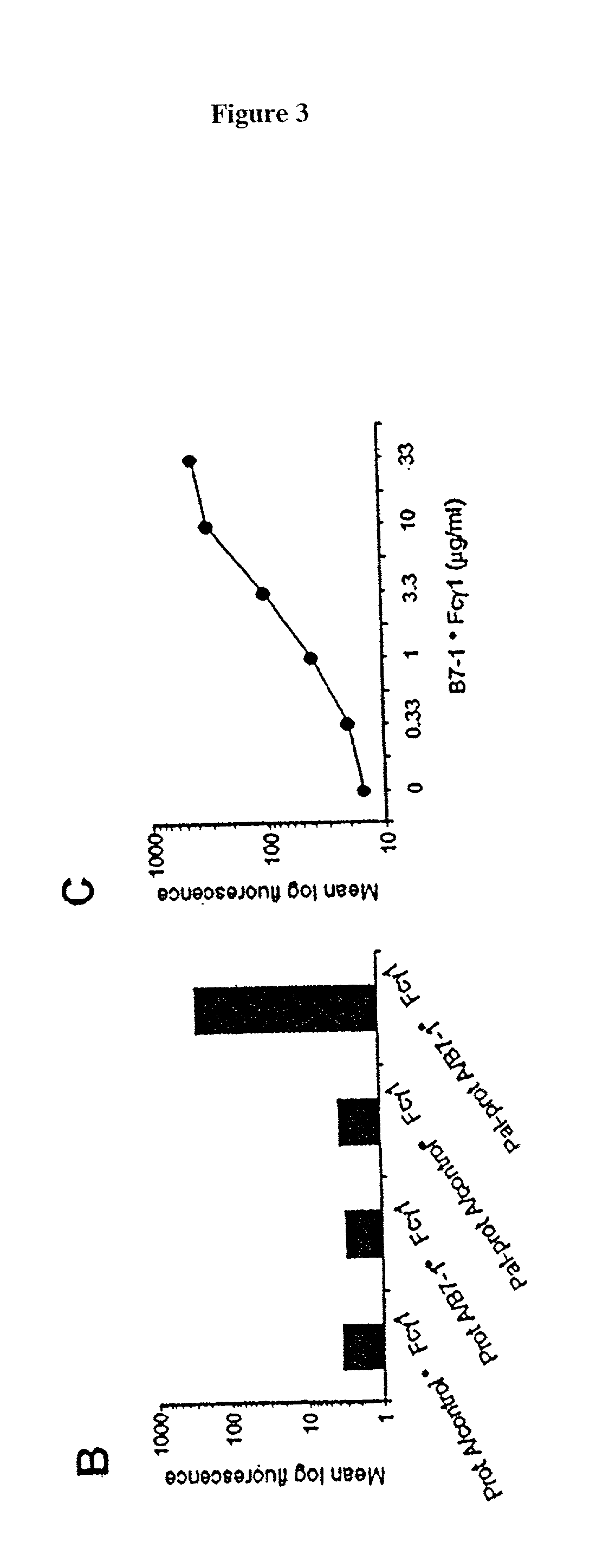
[0072] C3H / HeN mice, purchased from Harlen (USA), Indianapolis, were inmmunized with a cell vaccine generated from the T-50 cell line, obtained from Avranham Hochberg, Hadassah University Hospital. The vaccine was prepared following the procedure generally outlined in Example 1, using palmitated protein A and mB7-1.multidot.Fc, m4=1BBL.multidot.Fc, and hCD40L.multidot.Fc fusion proteins. Basically, the cells were coated with the lipidated protein A at 37.degree. C. at a ratio of 40 .mu.g protein A per 40.times.10.sup.6 cells. The cells were then incubated at 4.degree. with an equal mixture of the three fusion proteins at a ratio of 20 .mu.g total protein per 4.times.10.sup.7 cells. The cell vaccine was injected into the mice subcutaneously at a dose of 10.sup.6 cells per injection. The injections were given once a week and continued for three weeks. One week after the last injection, the animals were challenged with 10.sup.6 wild-type T-50 tumor cells, injected intradermally on the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com