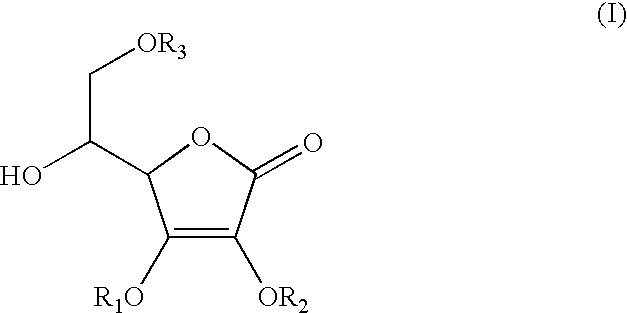
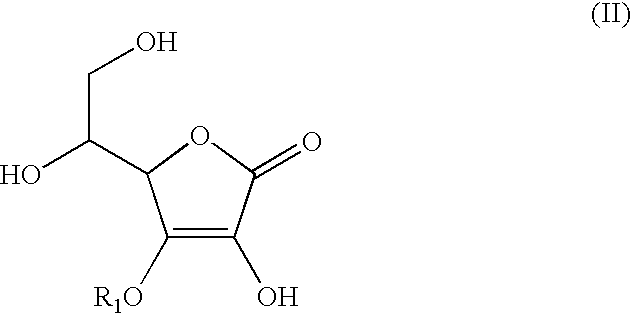
Anti-inflammation analgesic preparation
an anti-inflammation and preparation technology, applied in the direction of biocide, plant/algae/fungi/lichens, drug compositions, etc., can solve the problems of inability to meet the expectation of cyclooxygenase inhibitor indomethacin, no satisfactory anti-inflammation analgesic preparation which is free from side effects, and no technique to utilize ascorbic acid in an anti-inflammation analgesic preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 2
[0031]
3 L-3-0-dodecylascorbic acid 4% Ethanol 10% methyl salicylate 0.2% Hydrophilic ointment Balance
[0032] L-3-0-Dodecylascorbic acid and methyl salicylate were weighed, ethanol was added, and these were mixed. This solution and a hydrophilic ointment were mixed and totally homogenized to obtain a product.
preparation example 3
[0033]
4 L-3-0-Octadecyloxycarbonylmethylascorbic acid 5% Ethanol 10% Methylparaben 0.2% L-menthol 1.0% Hydrophilic ointment Balance
[0034] L-3-0-Octadecyloxycarbonylmethylascorbic acid and L-menthol were weighed, ethanol was added, and these were mixed. This solution and a hydrophilic ointment were mixed and totally homogenized to obtain a product.
preparation example 4
[0035]
5 L-3-0-Dodecylcarbonylmethylascorbic acid 5% Ethanol 10% Methylparaben 0.2% Thymol 1.0% xanthan gum 2.0%
[0036] L-3-0-Dodecylcarbonylmethylascorbic acid, methylparaben and thymol were weighed, ethanol was added, and these were mixed. This solution was added to a xanthan solution, and these were mixed and totally homogenized to obtain a product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| swelling | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com