Method and reagent for treatment of diseases caused by expression of the c-myc gene
a technology of c-myc gene and reagent, which is applied in the field of method and reagent for treating diseases caused by expression of the cmyc gene, can solve the problems of destroying the ability to direct the synthesis of encoded proteins, and achieves the effects of prohibitive therapeutic cost, reduced treatment cost, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The drawing will first briefly be described.
[0025] Drawing
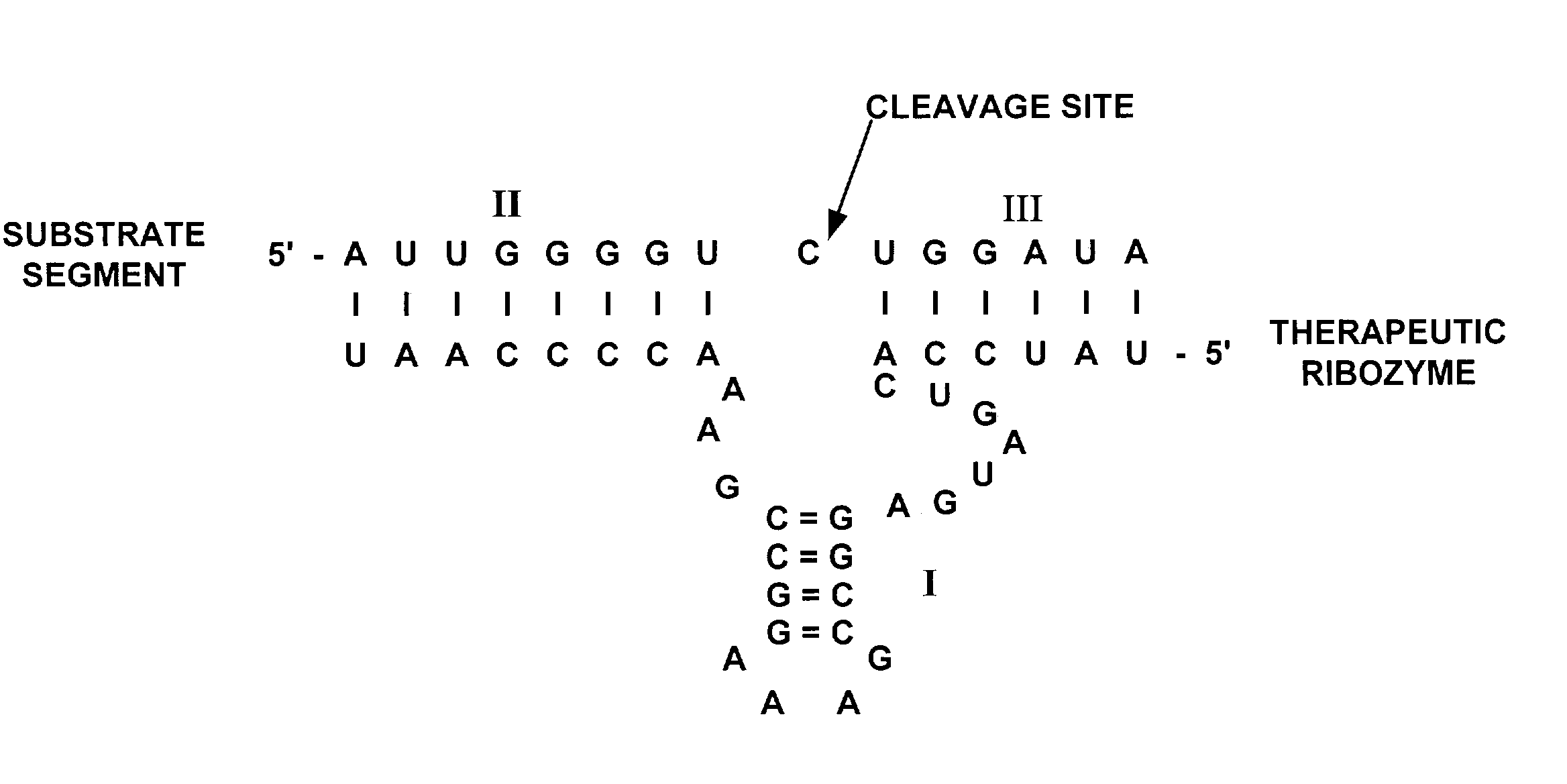
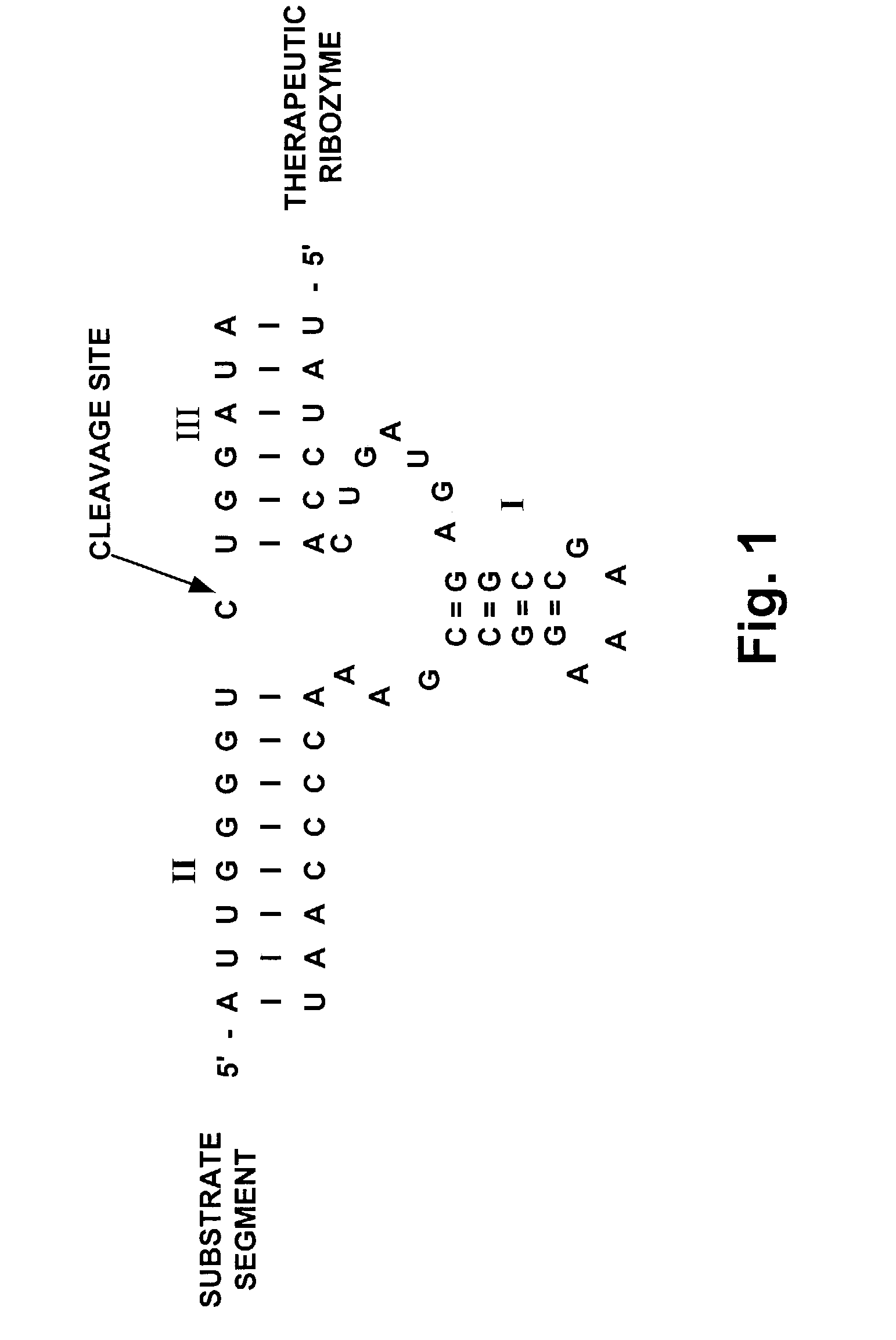
[0026] FIG. 1 is a diagrammatic representation of a hammerhead motif ribozyme showing stems I, II and III (marked (I), (II) and (III) respectively) interacting with a viral target region. The 5' and 3' ends of both ribozyme and target are shown. Dashes indicate base-paired nucleotides.
[0027] Target Sites
[0028] Ribozymes targeting selected regions of mRNA associated with tumor cell growth are chosen to cleave the target RNA in a manner which preferably inhibits translation of the RNA. Genes are selected such that inhibition of translation will preferably inhibit cell replication, e.g., by inhibiting production of a necessary protein. Selection of effective target sites within these critical regions of MRNA entails testing the accessibility of the target RNA to hybridization with various oligonucleotide probes. These studies can be performed using RNA probes and assaying accessibility by cleaving the hybrid molecule with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com