Implantable device for monitoring aneurysm sac parameters
a technology of aneurysm and parameters, which is applied in the field of implantable devices for monitoring aneurysm sac parameters, can solve the problems of aneurysm formation, endanger the health of a patient, and death of the patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
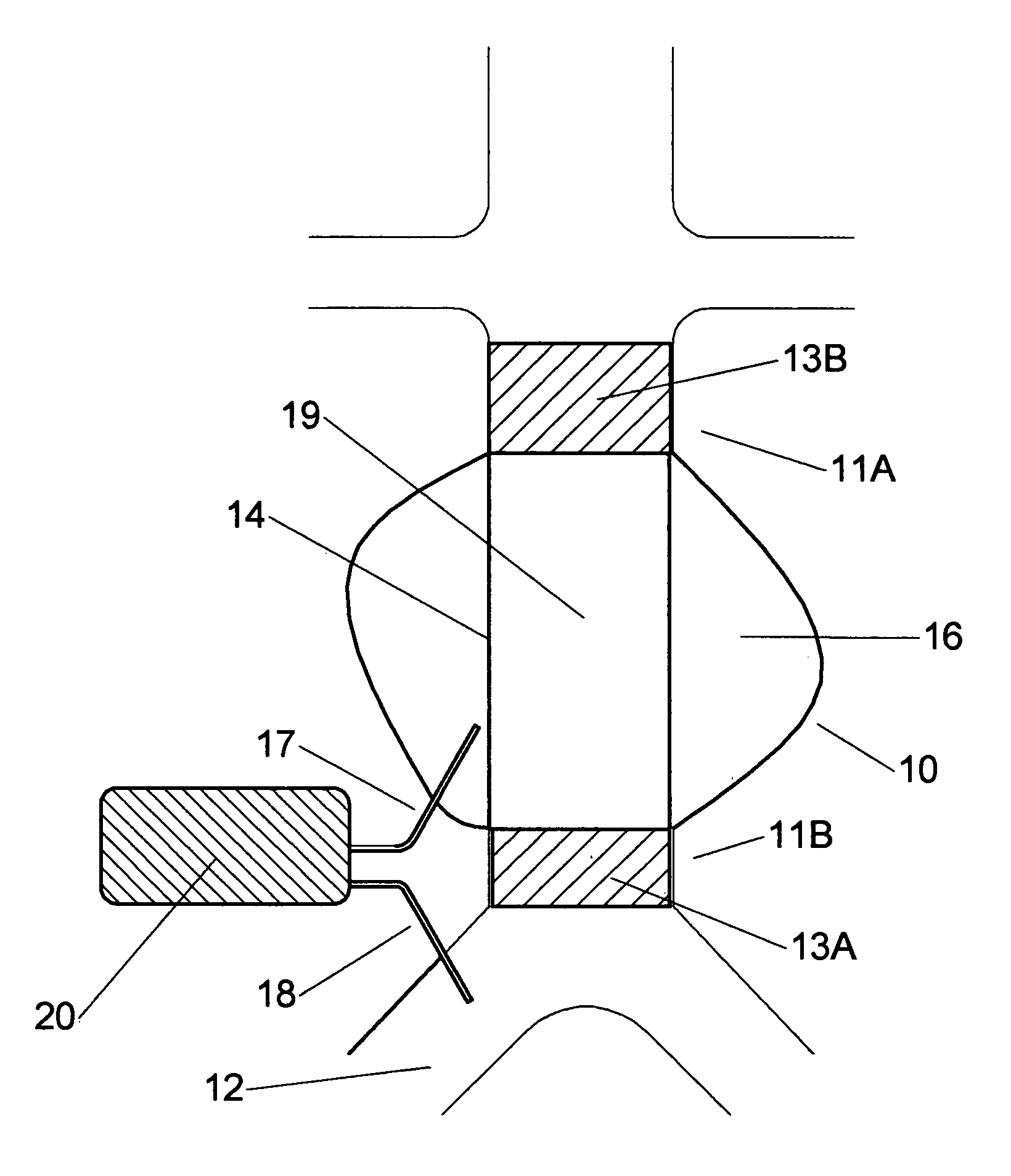
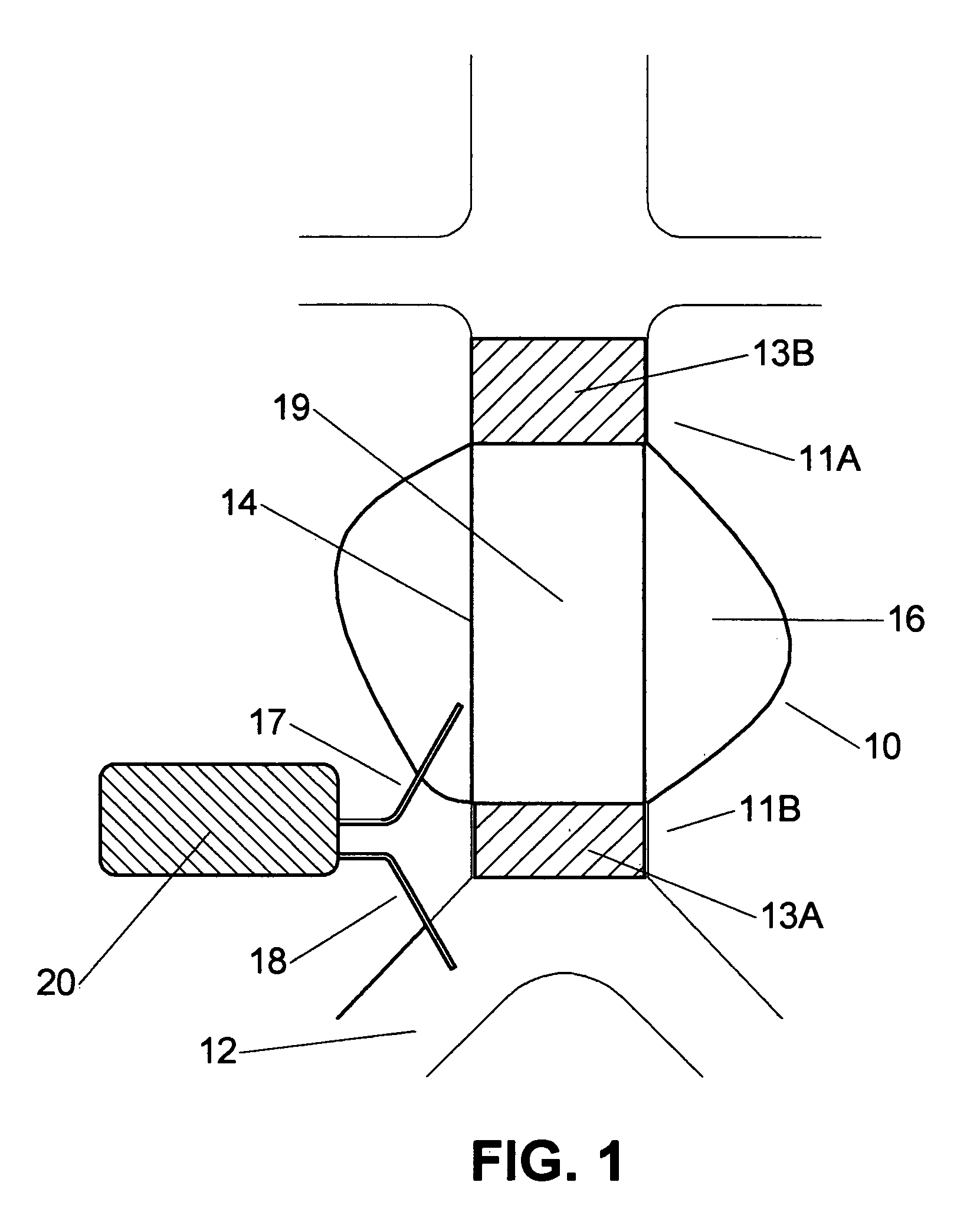
[0041] An aneurysm is dangerous to the health of a human since rupture of an aneurysm would lead to internal bleeding with possible lethal consequences. In order to negate this risk endoprosthesis are used for bridging the aneurysm. In FIG. 1 a tube endoprosthesis 19 is shown, bridging aneurysm 10. The endoprosthesis 19 comprises a flexible closed wall 14, and is provided fully or at both ends with a stent 13A, 13B. A first end of the endoprosthesis 11A is positioned at the upstream side of the aneurysm 10, by means of the stent 13B, a second end 11B at the opposite, downstream side of the aneurysm 10 by means of the second stent 13A. Endoprostheses of this type are known in the state of the art and are for example manufactured under the registered trademarks Ancure by Guidant Corporation, Zenith by Cook Corporation, and Excluder by WL Gore. However, all kinds of endoprosthesis can be used, for example a tube, bifurcated, unibody, or bilateral prosthesis.
[0042]FIG. 1 shows a first ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com