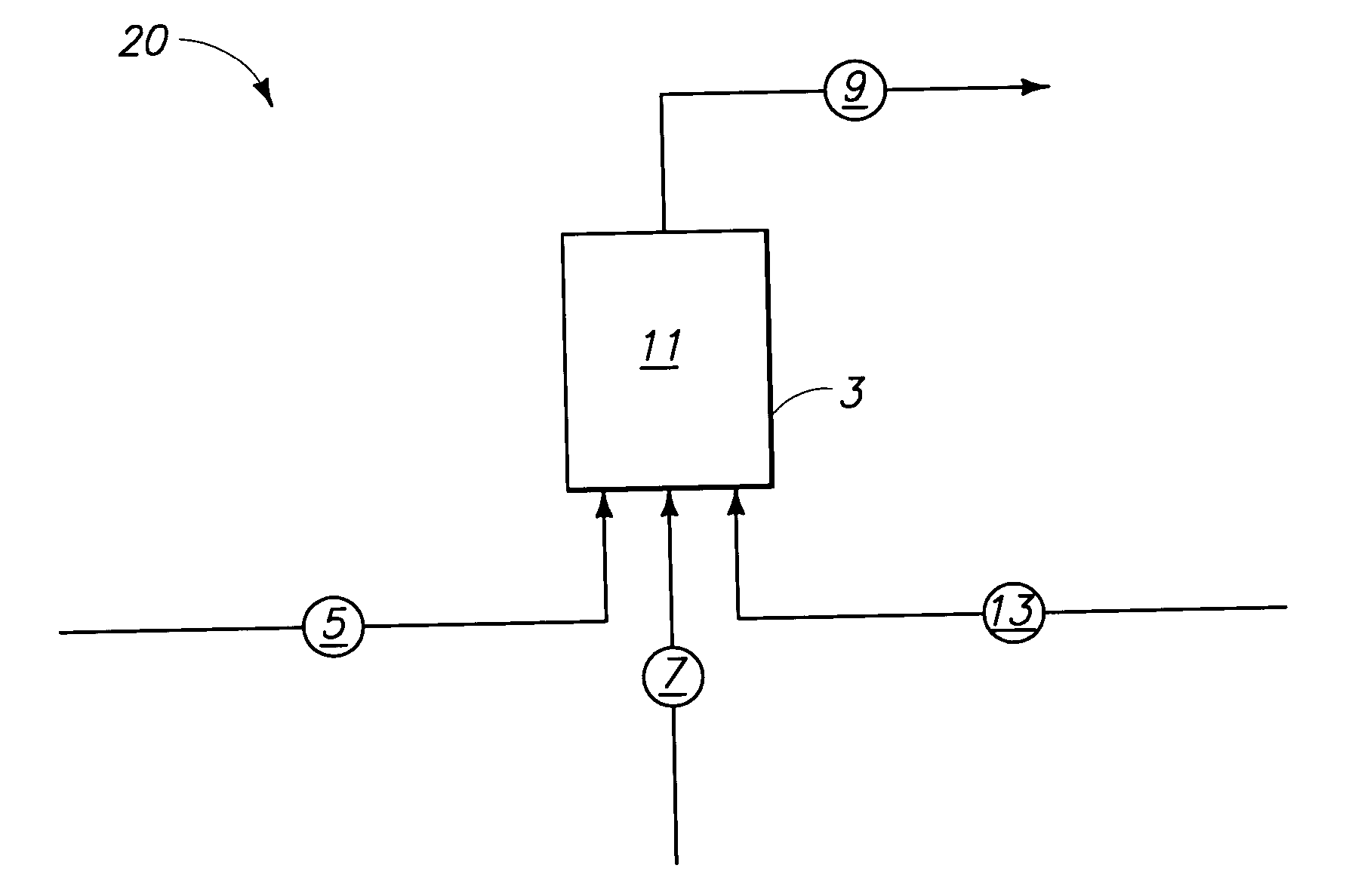
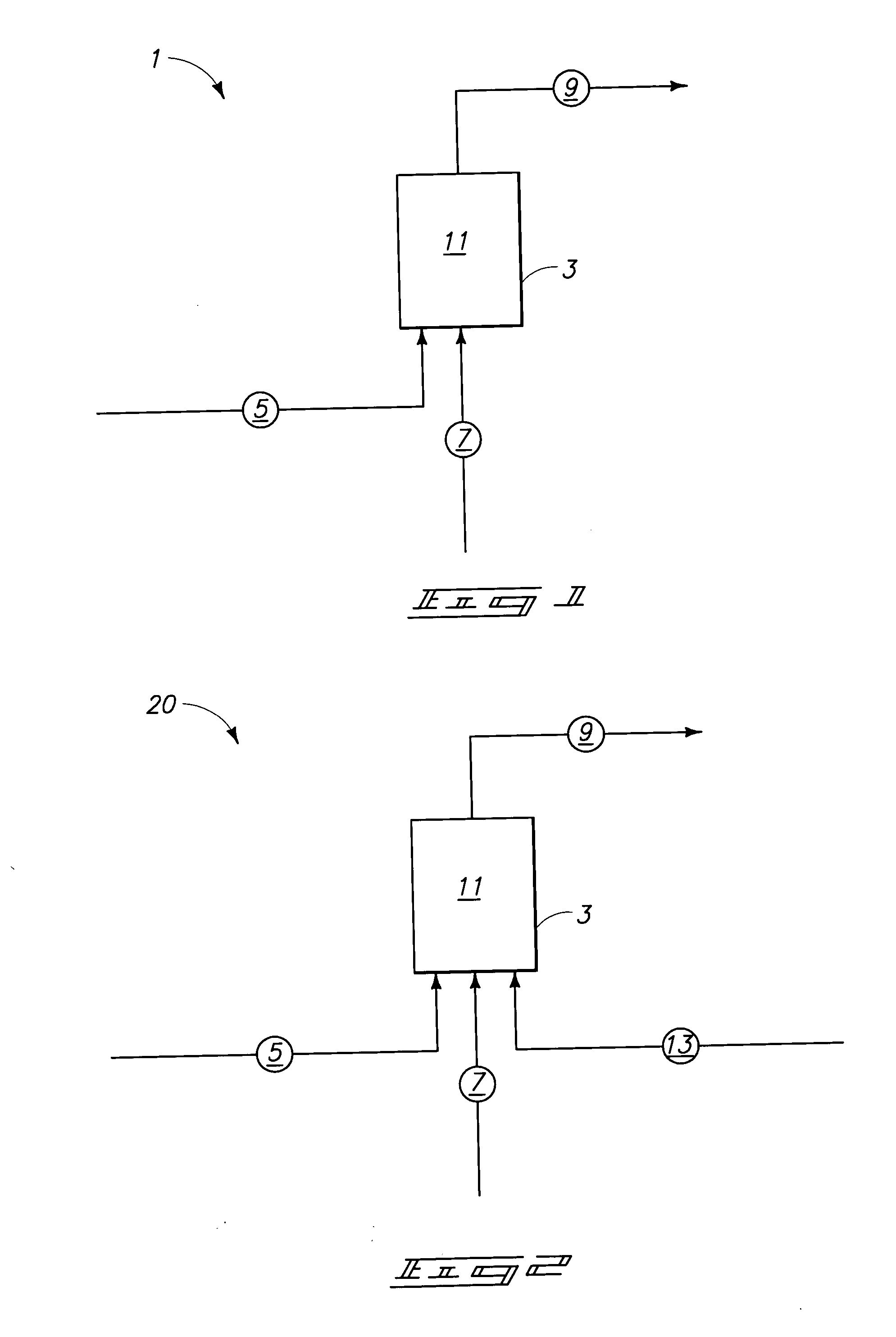
Systems and methods for producing fluorocarbons
a technology of fluorocarbons and production methods, applied in the field of fluorocarbon production, can solve the problem that known methods do not readily translate into predictable applications for other compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Potassium Catalysts
[0047]
TABLE 1CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% KClA20011.56.419.346.437.283.610% KClA25018.73.945.652.019.871.910% KClA30014.5363.913.757.170.8
[0048]
TABLE 2CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% KFA30010.64.16.96.265.972.110% KFA2509.74.47.228.166.674.710% KFA3009.67.114.049.327.576.810% KFA3503.45.259.629.531.761.110% KFA4007.95.638.724.720.455.0
example 2
Zirconium Catalysts
[0049]
TABLE 3CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% ZrCl2A20011.54.125.3138.056.194.110% ZrCl2A25010.38.266.1846.136.182.310% ZrCl2A3009.56.836.4338.936.675.510% ZrCl2A3508.87.393.6318.747.466.1
[0050]
TABLE 4CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% ZrCl4B20012.312.722.9527.821.048.810% ZrCl4B25011.315.441.4246.025.371.310% ZrCl4B30010.522.676.9629.935.565.510% ZrCl4B3509.211.495.1918.544.262.7
[0051]
TABLE 5CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% CuZrCl2B20012.211.818.2430.661.391.910% CuZrCl2B25011.111.640.4159.828.788.510% CuZrCl2B3009.211.240.4749.335.684.910% CuZrCl2B3509.413.337.646.636.983.510% CuZrCl2B4007.611.831.2428.052.880.910% CuZrCl2B5007.614.731.844.032.036.1
example 3
Tungsten Catalysts
[0052]
TABLE 6CatalystCat.Temp.Contact TimeH2 / CFC-217% SelectivityPrecursorPrep.(° C.)(sec)Mole Ratio% ConversionHFPHFC-227Total10% NaWA20012.110.323.144.540.384.710% NaWA2501111.549.9856.025.881.810% NaWA3001010.195.1940.227.087.210% NaWA3509.19.794.9830.528.358.8
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com