CDR grafted type III anti-CEA humanized mouse monoclonal antibodies
a humanized mouse and monoclonal antibody technology, applied in the field of immunological reagents, can solve the problems of limiting the diagnostic and therapeutic utility of some of the reagents so far developed, limiting the diagnostic or therapeutic agent from reaching the target site, and reducing the effective concentration of the targeting agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Culturing Antibody Producer Cells
[0082] A mouse / mouse hybridoma cell line producing Class III, anti-CEA monoclonal antibodies was established according to Hansen et al. (1993) above and Primus et al. (1983) above.
[0083] Cells were selected for secretion of kappa IgG1 by testing conditioned medium using standard isotyping techniques. A variety of kits for this purpose are commercially available. Such cells were screened for production of antibody by testing conditioned medium using a standard blocking assay described above. Stocks of producer cells that proved out in the assay were expanded and frozen in liquid nitrogen.
example 2
Isolating RNA from Producing Cell Lines
[0084] MN-14-producing cells were expanded in culture, collected by centrifugation and washed. Total RNA was isolated from the cells in the pellet according to Favaloro et al., Methods in Enzymology 65: 718 (1980) and Orlandi et al., Proc. Nat'l Acad. Sci., USA 86: 3833 (1989), which are incorporated by reference.
example 3
cDNA Synthesis and Amplification of the Heavy Chain Variable Region
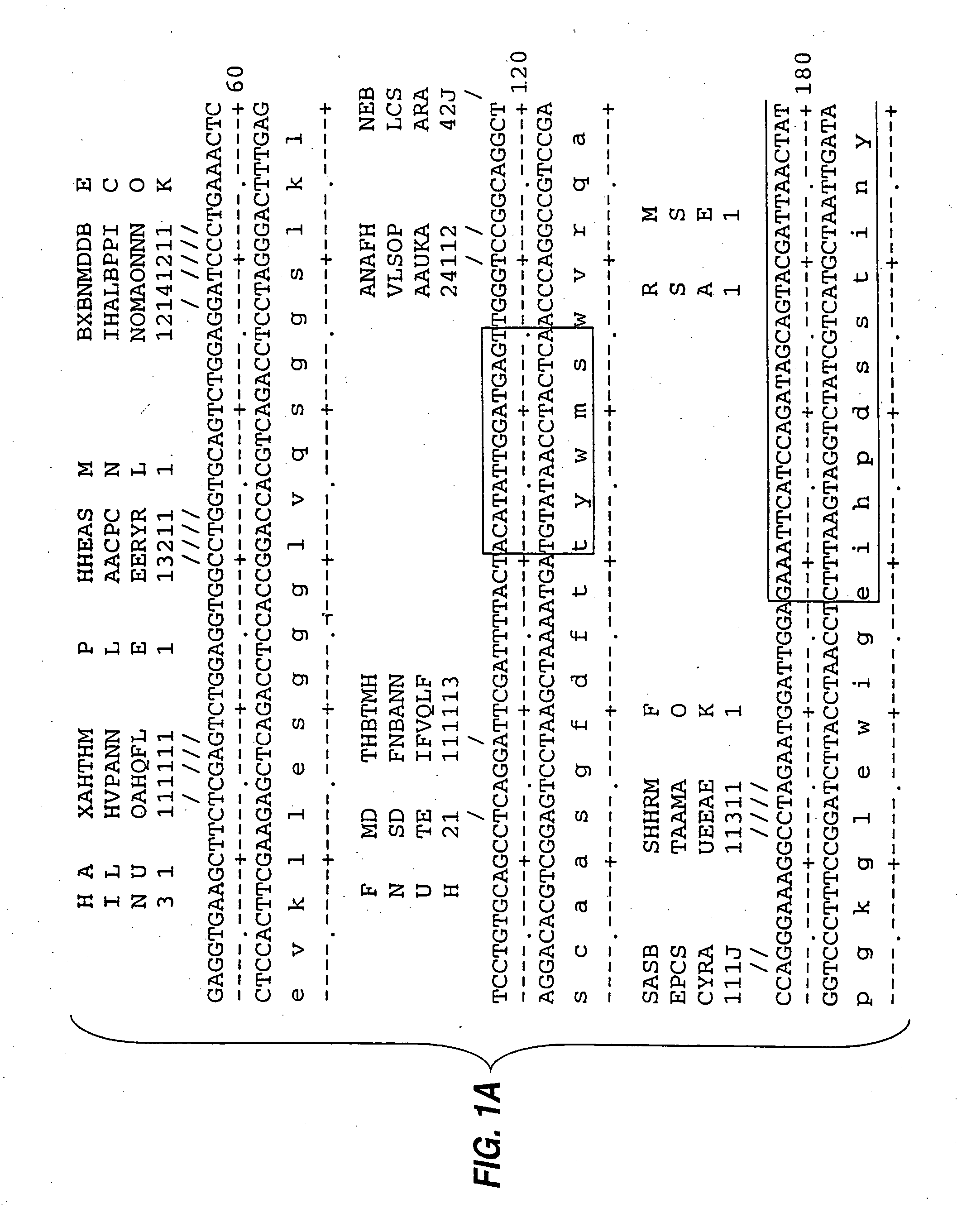
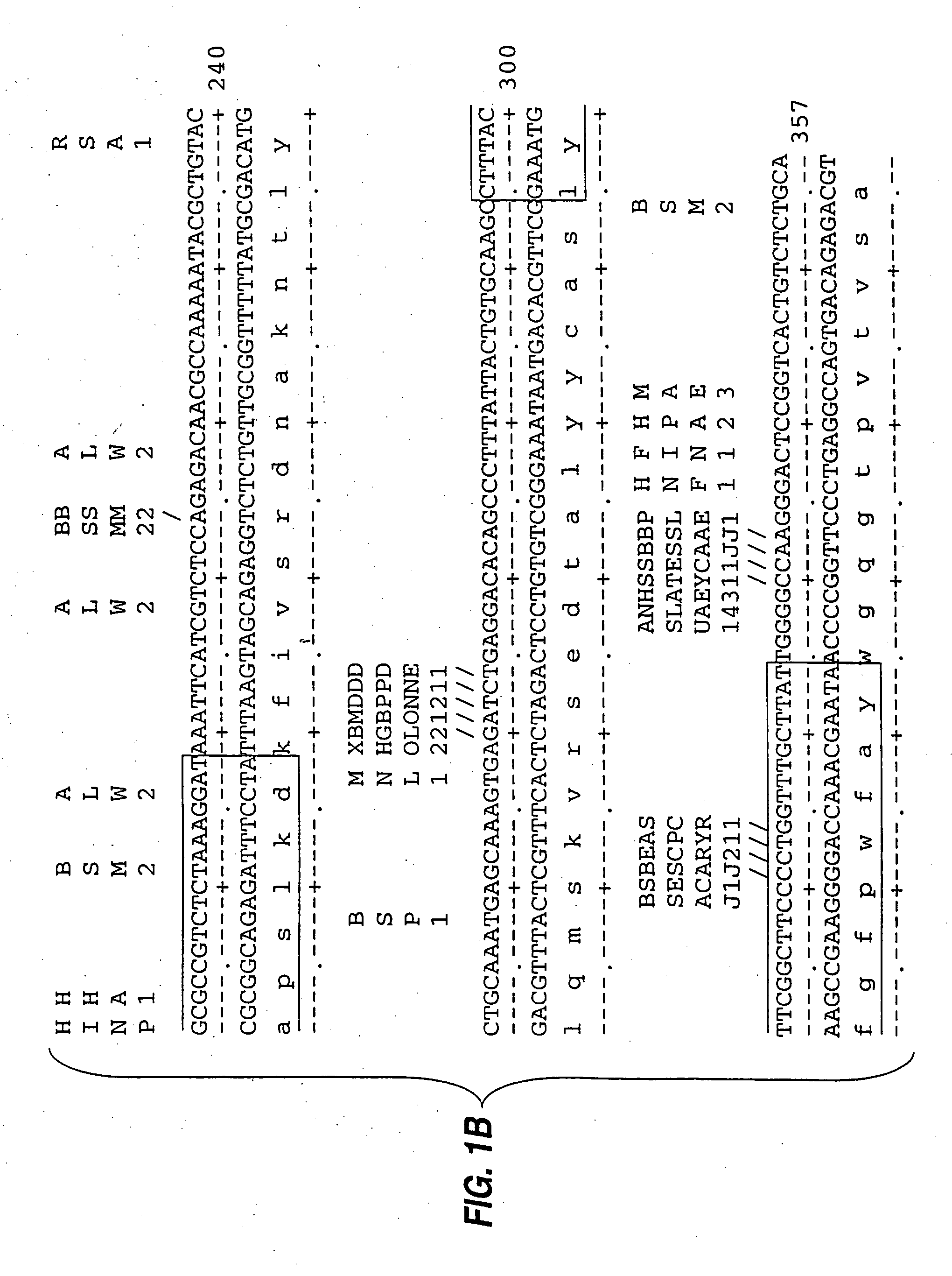
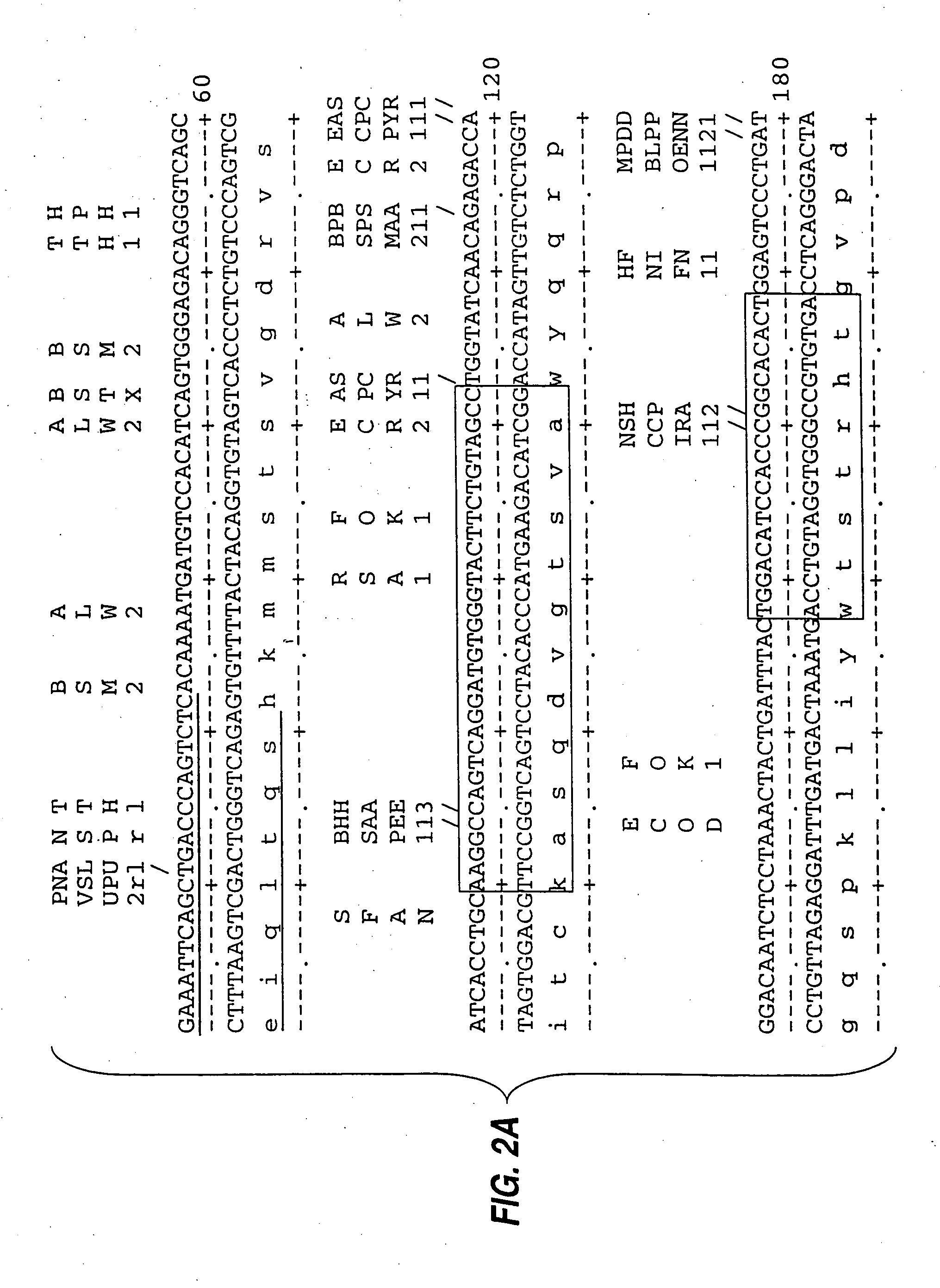
[0085] mRNA from MN-14 producing cells was used to synthesize cDNA using standard techniques of cDNA synthesis and DNA amplification by PCR, as described below. In general, the primers used for PCR included a restriction endonuclease cleavage site at their 5′ ends to facilitate cloning of the amplification product. An oligonucleotide complementary to the end of the sense strand of the DNA encoding the first constant region domain of the murine IgG1 heavy chain (“CH1”) was used to prime first strand cDNA synthesis by reverse transcriptase. The sequence of this primer, CG1FOR, is shown in Table 1. Table 1 below provides other oligonucleotide sequences used herein.
TABLE 1OLIGONUCLEOTIDE SEQUENCESSEQ.IDNO.CG1FOR415′ GGAAGCTTAGACAGATGGGGGTGTCGTTTTG 3′VH1FOR425′ TGAGGAGACGGTGACCGTGGTCCCTTGGCCCCAG 3′VH1BACK435′ AGGTSMARCTGCAGSAGTCWGG 3′SH1BACK445′ TGGAATTCATGGRATGGAGCTGGRTCWTBHTCTT 3′SH2BACK455′ TGGAATTCATGRACTTCDGGYTCAA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com