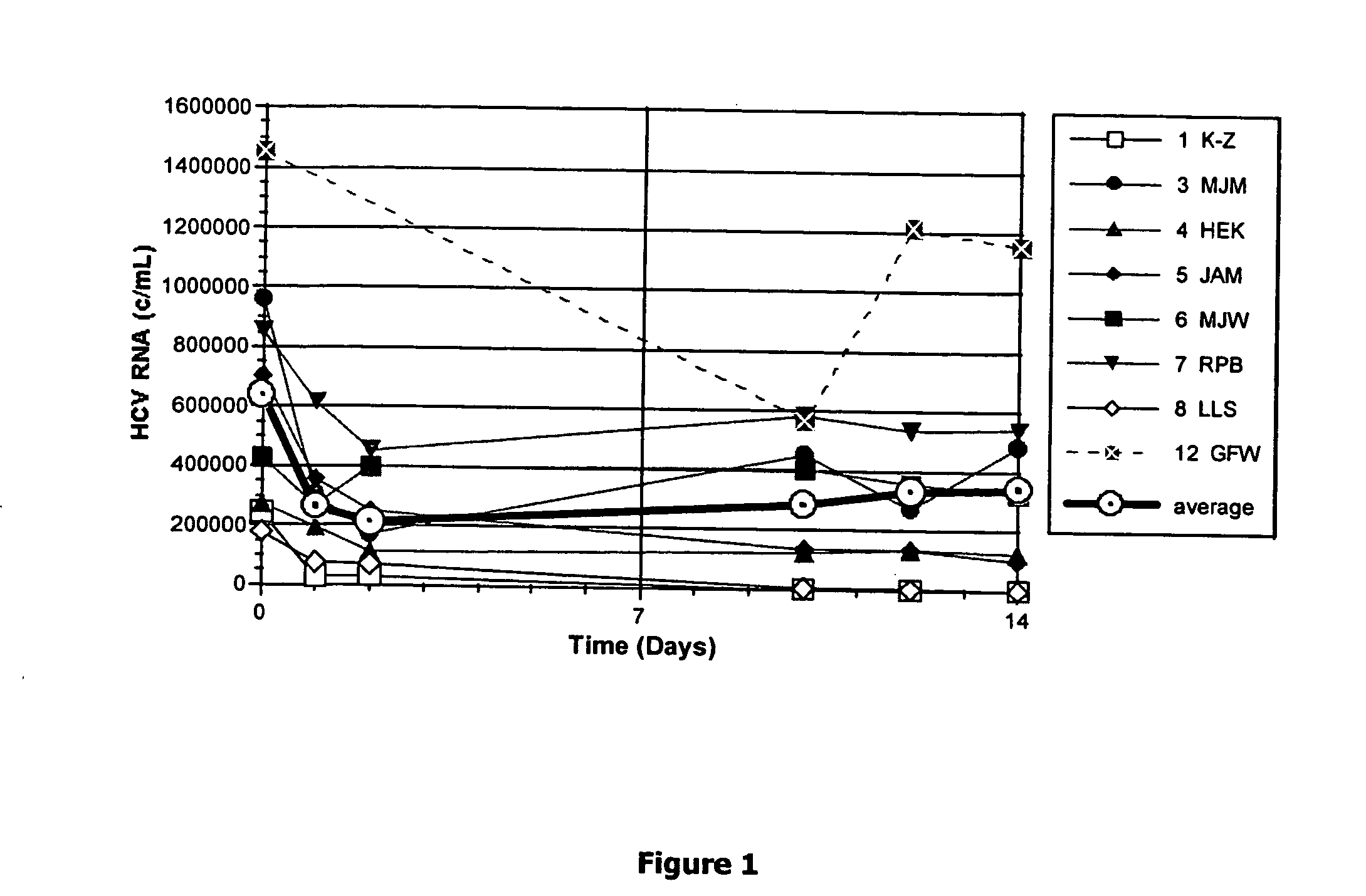
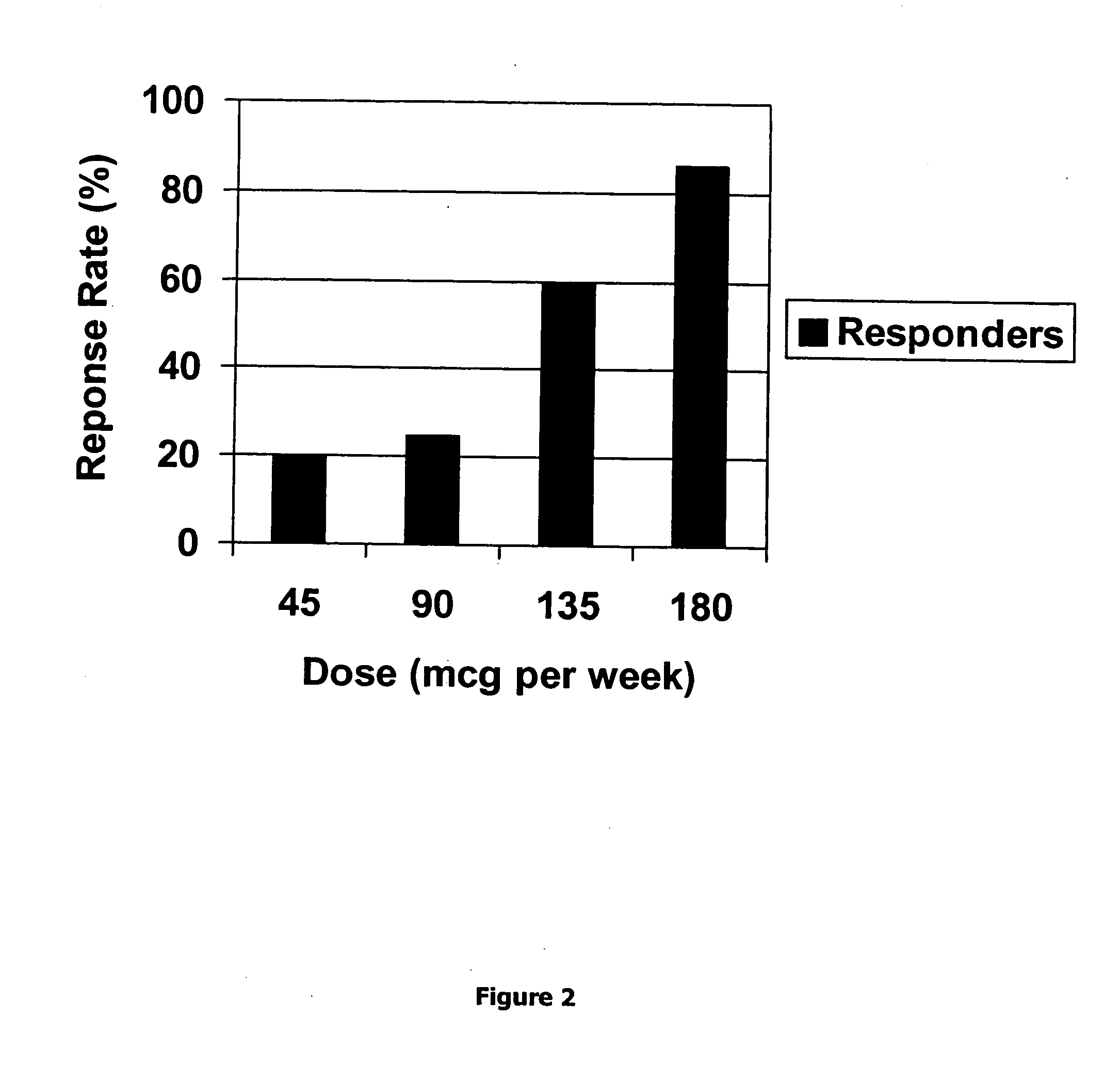
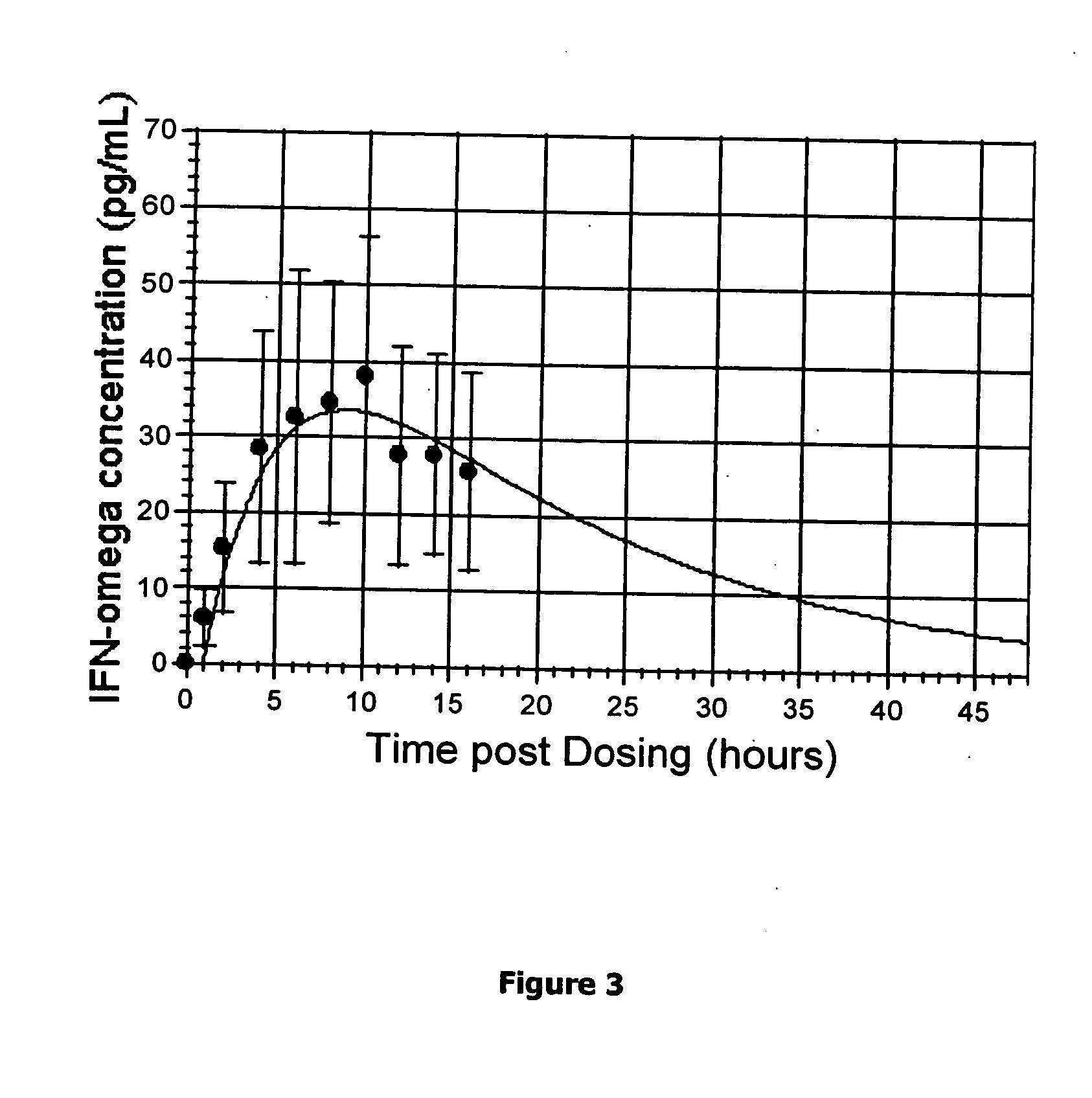
Method for short-term and long-term drug dosimetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0141] Omega Interferon in a Short-Term Formulation Followed by Omega Interferon in a Long-Term Formulation Suitable for Use in an Implantable, Non-Erodible Drug Delivery Formulation
Preparation and Administration of Omega Interferon in a Short-Term Formulation
[0142] Omega interferon is produced by standard genetic engineering techniques in E. coli bacteria or in mammalian Chinese hamster ovary cells. Such techniques are further described for interferons generally in U.S. Pat. No. 4,727,138 and more specifically for omega interferon in U.S. Pat. No. 5,120,832 and U.S. Pat. No. 5,231,176. The interferon is then purified and used immediately or frozen and then subsequently thawed for use. The interferon may be lyophilized with appropriate stabilizers for subsequent reconstitution with water-for-injection or other suitable solvent or the interferon may be prepared for use initially as a liquid formulation.
[0143] For a lyophilized preparation of omega interferon 33 μg of omega interf...
example 2
[0155] Omega Interferon in a Short-Term Formulation Followed by Omega Interferon in a Long-Term Formulation Suitable for Use in an Implantable or Injectable Erodible or Dispersible Drug Delivery System
[0156] Omega interferon is prepared for short-term use as described in EXAMPLE 1.
[0157] Erodible or dispersible implantable or injectable drug delivery systems suitable for use in the long-term delivery of an interferon, including omega interferon, include such systems as described in U.S. Pat. No. 5,543,156, which is incorporated herein by reference, as well as in U.S. Pat. Nos. 5,529,914, 5,858,746, and 6,129,761, which are also incorporated herein by reference.
[0158] Those skilled in the arts will recognize that the current invention can be utilized to optimize or improve the long-term treatment of warm-blooded animals with any interferon-responsive condition and with any interferon or interferon-like molecule suitable for short-term formulation or suitable for long-term formulat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com