Vitro maturation of immature human oocytes
a technology of immature human oocytes and vitro maturation, which is applied in the field of assisted reproductive technology, can solve the problems of ovulation hyperstimulation syndrome (ohss), not all ovulations have matured to the m-ii stage, and the procedure of ovarian stimulation requires frequent blood sampling and ultrasound monitoring, and/or death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Source of Immature Oocytes
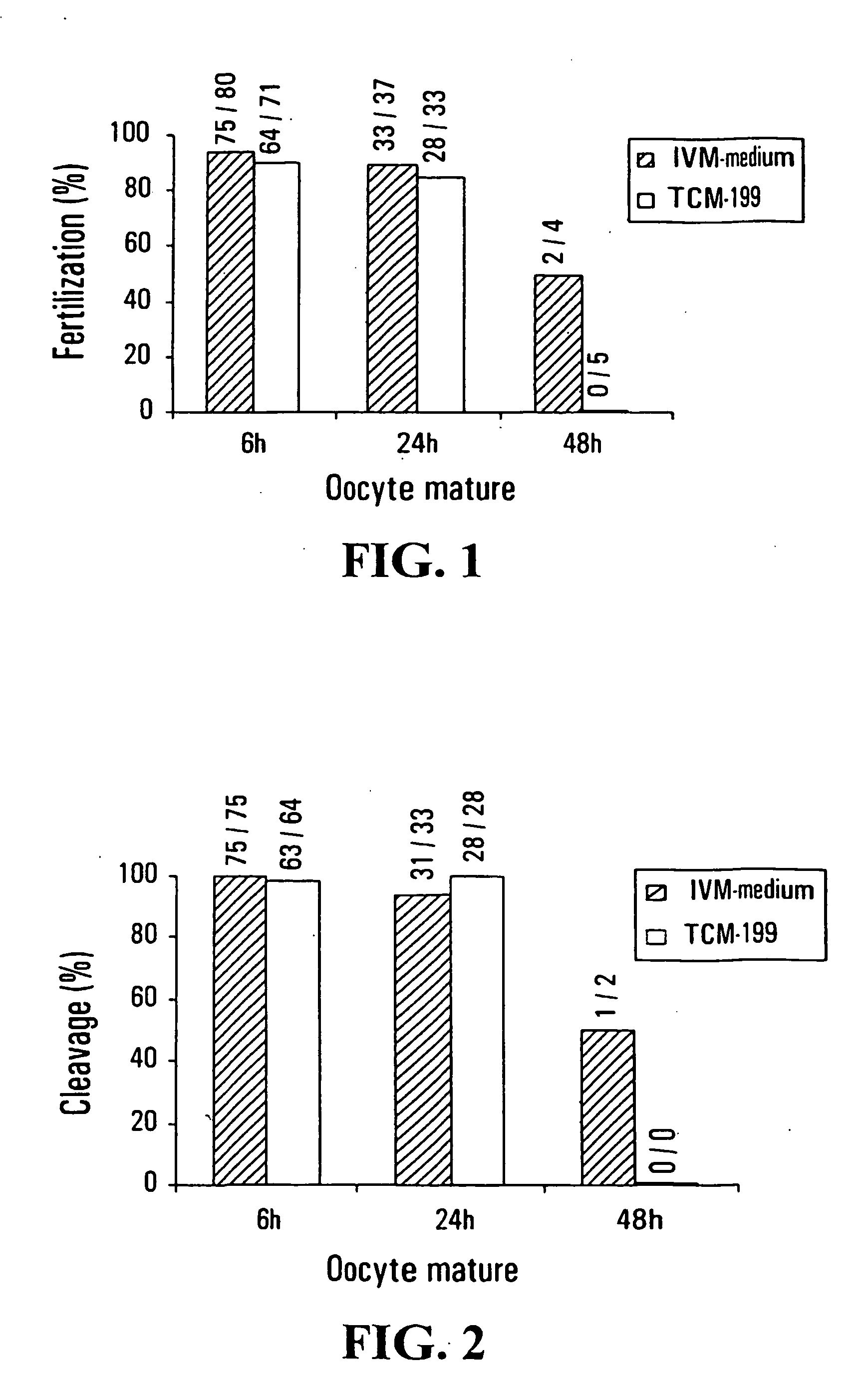
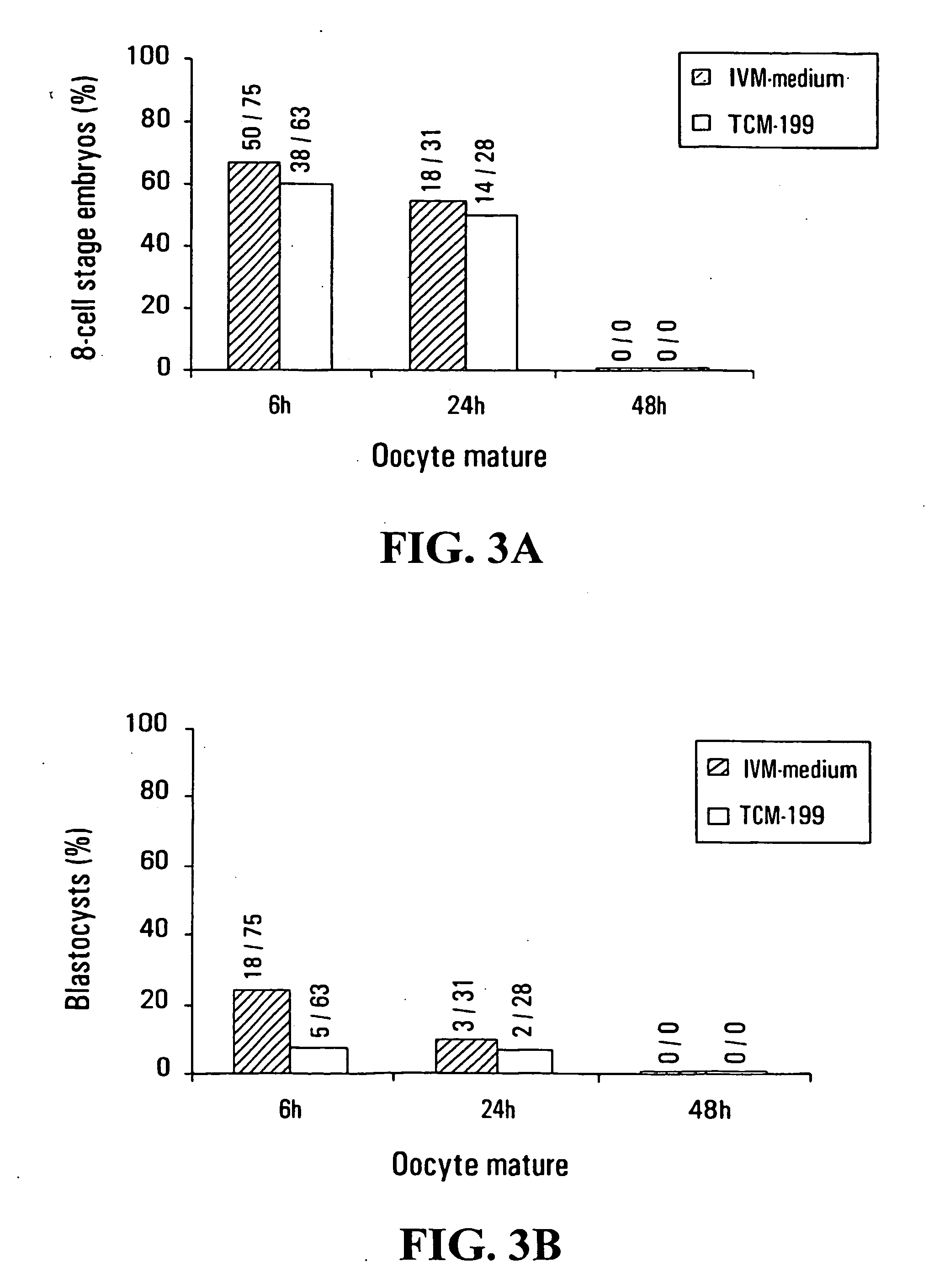
[0108] A total of 521 immature oocytes (213 oocytes at GV stage and 308 oocytes at M-I stage) were collected from 183 women (age range between 28 to 40 years) who underwent controlled ovarian stimulation and ICSI cycles under informed consent. Ovarian stimulation was performed with GnRHa, HMG or FSH and HCG using standard protocols.
[0109] 36 hours after HCG injection, oocyte retrieval was carried out using an ultrasound-guided trans-vaginal probe. The collected COC were cultured in human tubal fluid medium (HTF; Irvine Scientific) supplemented with 10% synthetic serum substitute (SSS; Irvine Scientific) and placed in a 5% CO2 incubator (37° C. with high humidity) for at least 1 h before they were stripped from their cumulus cells.
[0110] COC were stripped with 85 IU / ml hyaluronidase in HTF medium and mechanical pipetting. All oocytes were completely denuded from their cumulus cells. Denuded oocytes were observed under an inverted microscope to assess for...
example 2
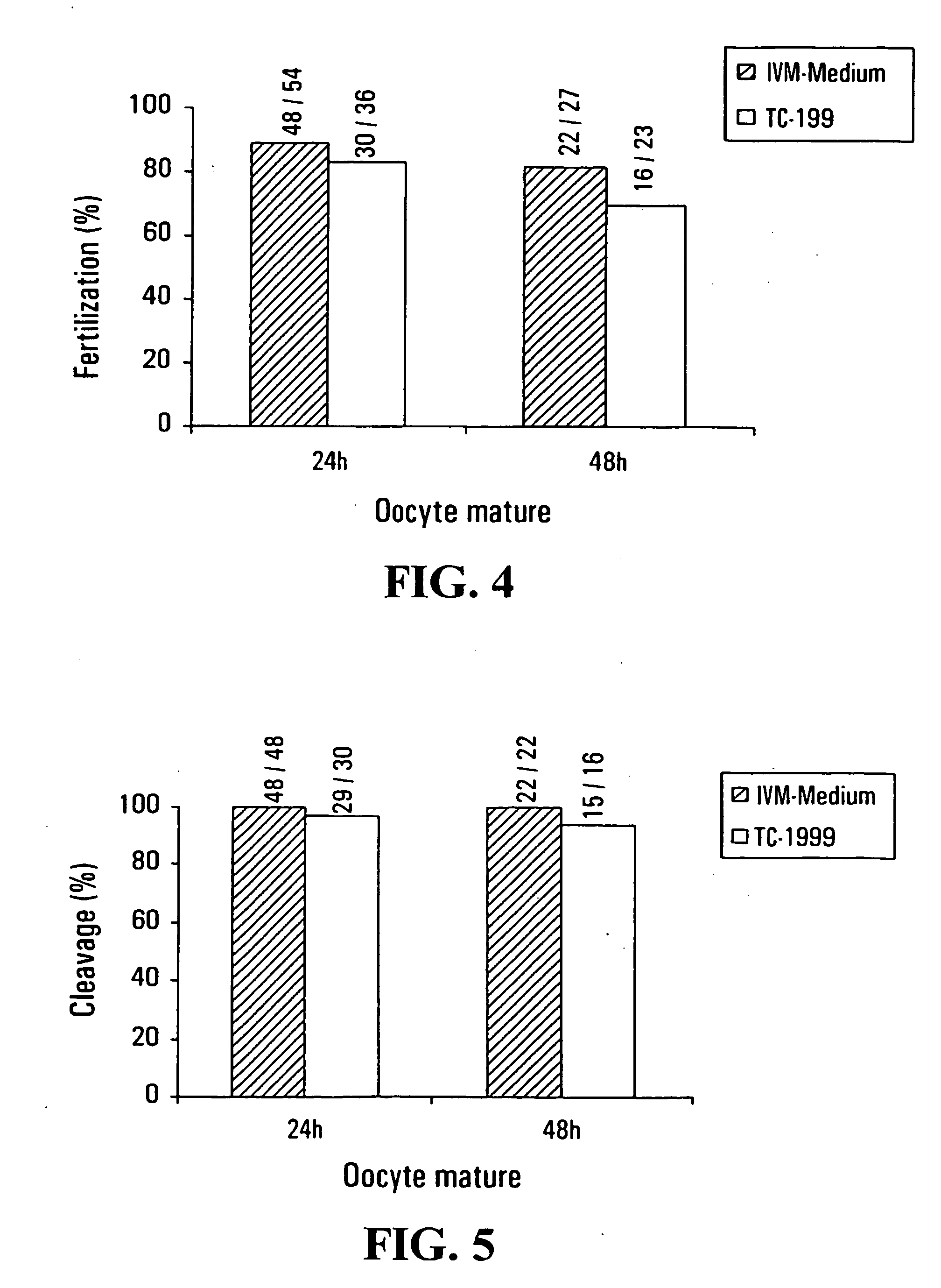
[0135] A total of 40 women with PCOS underwent 40 completed IVM treatment cycles. All patients were under 40 years of age (mean 32.3±3.9) and some patients presented irregular menstrual cycles or anovulation. All patients had a minimum 2-year history of infertility.
[0136] If the patients had irregular menstrual cycles, the treatment cycles was initiated by the administration of intra-vaginal progesterone (Prometrium; Schering, Pointe-Clair, Quebec, Canada) in a dose of 300 mg daily for 10 days. Withdrawal bleeding occurred within 3 days after the last dose.
[0137] On day 2 or 3 following the onset of menstrual bleeding, the patients underwent a baseline ultrasound scan to ensure that no ovarian cysts were present. Transvaginal ultrasound scans were repeated on day 8 to exclude the development of a dominant follicle. The size of all follicles on ultrasound scan had to be <10 mm in diameter on day 8 of the cycle.
[0138] Oocyte retrieval was performed on day 10 to 14 of the cycle. Bef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com