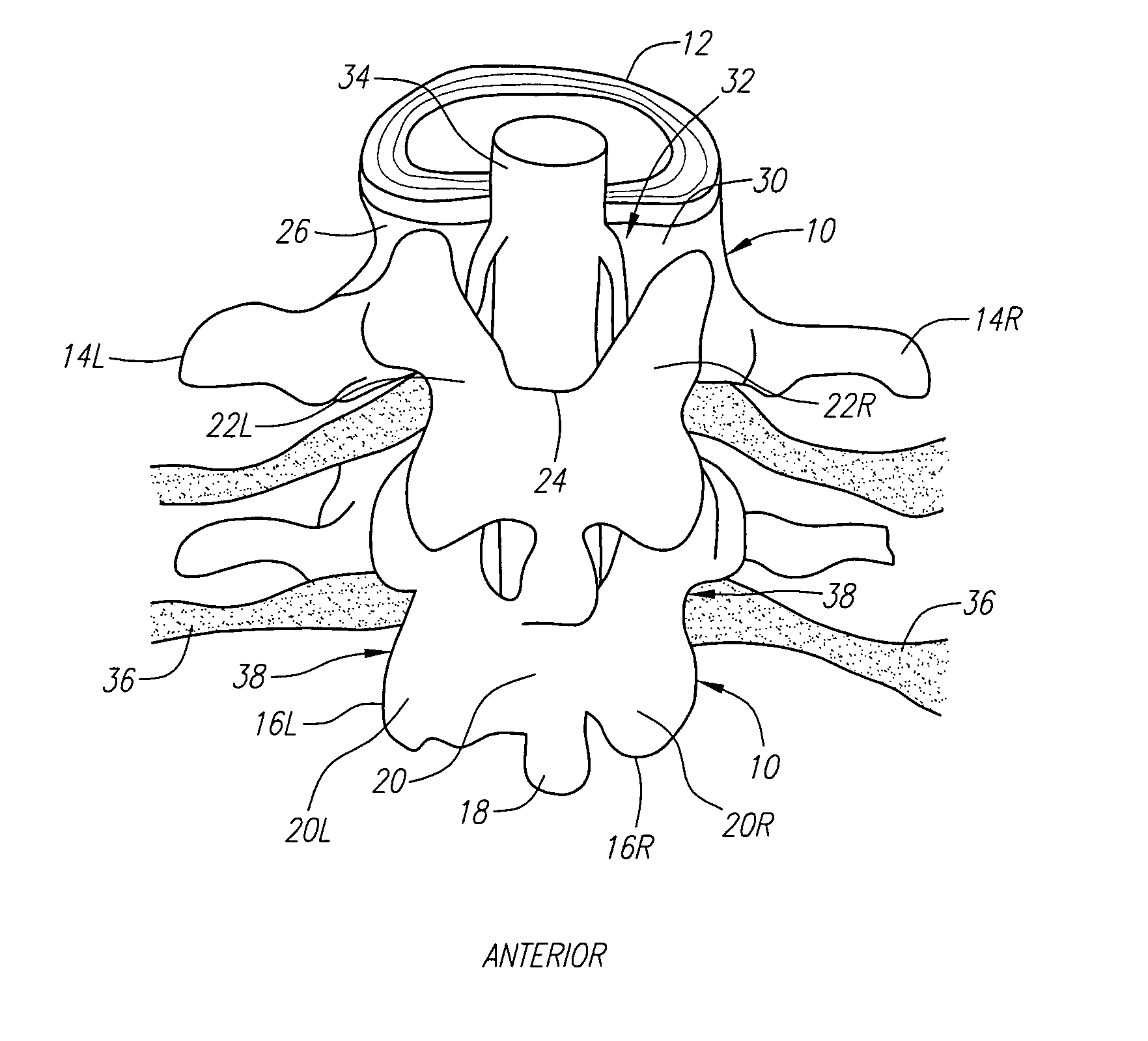
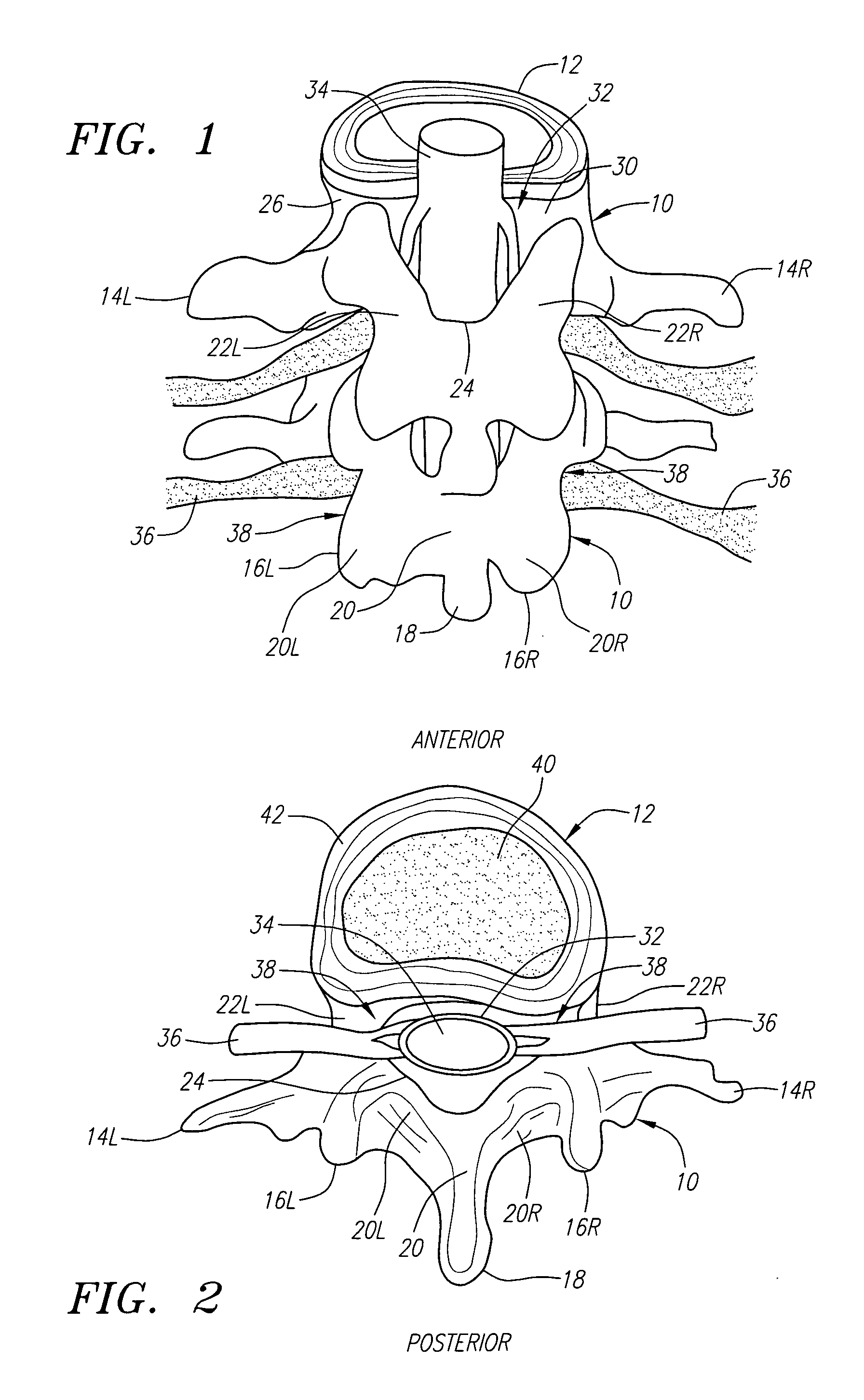
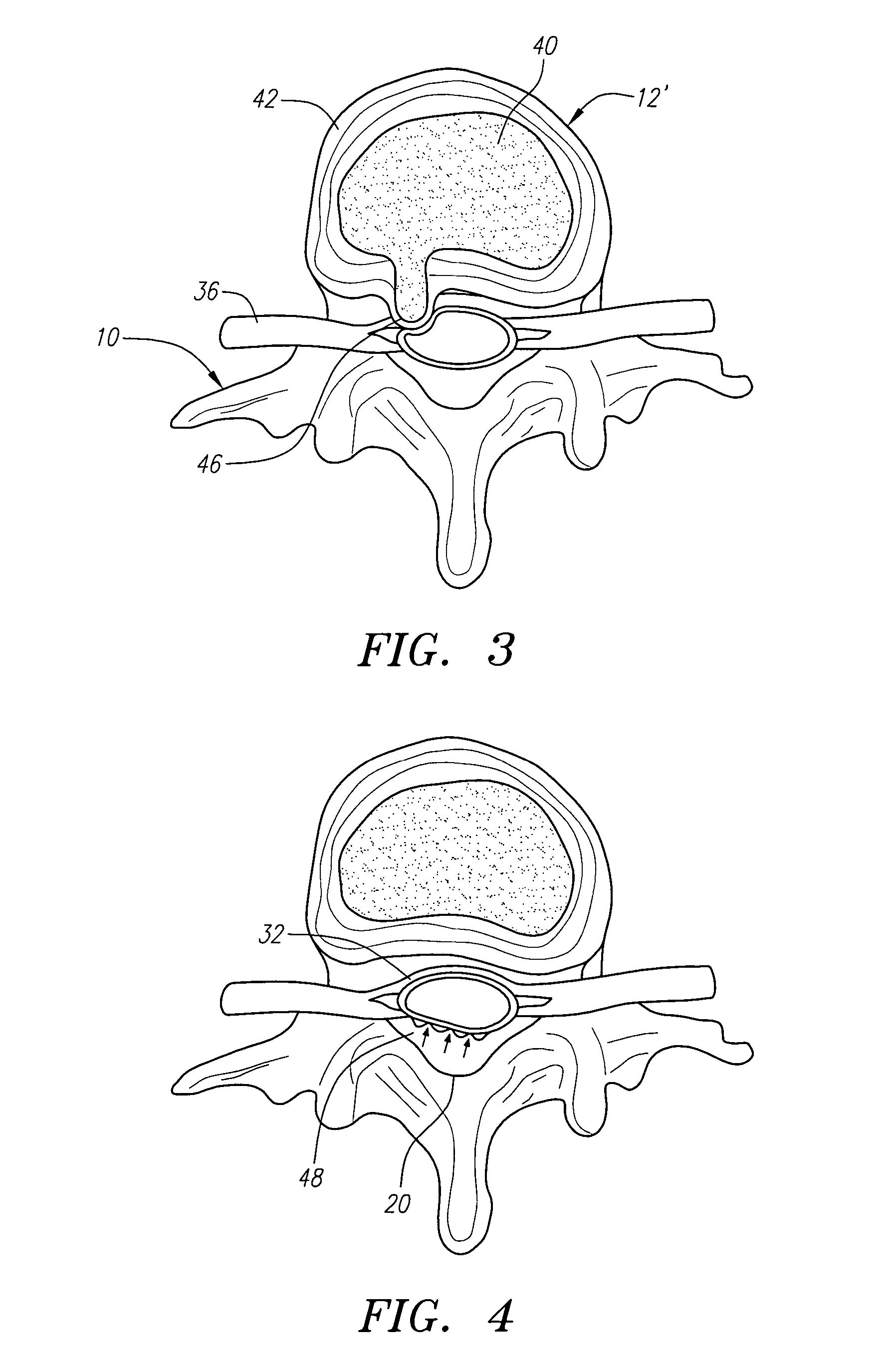
Radially adjustable tissue removal device
a tissue removal device and radial adjustment technology, applied in the field of medical devices and methods for removing tissue, can solve the problems of disc tissue irreparably damaged, pain, and even prolonged disability, and achieve the effect of facilitating its introduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0066]FIG. 8 illustrates a tissue removal system 100 constructed in accordance with a preferred embodiment of the present inventions. The system 100 generally comprises a tissue removal probe assembly 102 and a rotary drive unit 104 connected to the probe assembly 102 via a drive cable 106. The drive unit 104 may take the form of a standard rotary drive used for powering medical cutting instruments. The tissue removal probe assembly 102 comprises a cannula 108 and a tissue removal probe 110 disposed therein.
[0067] The cannula 108 comprises a shaft 112 having a distal end 114 and proximal end 116, a lumen 118 (shown in phantom) terminating in an exit port 120 at the distal end 114 of the cannula shaft 112, and a handle 122 mounted on the proximal end 116 of the cannula shaft 112. To facilitate introduction through tissue, the cannula shaft 112 is preferably stiff (e.g., it can be composed of a stiff material, or reinforced with a coating or a coil to control the amount of flexing), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com