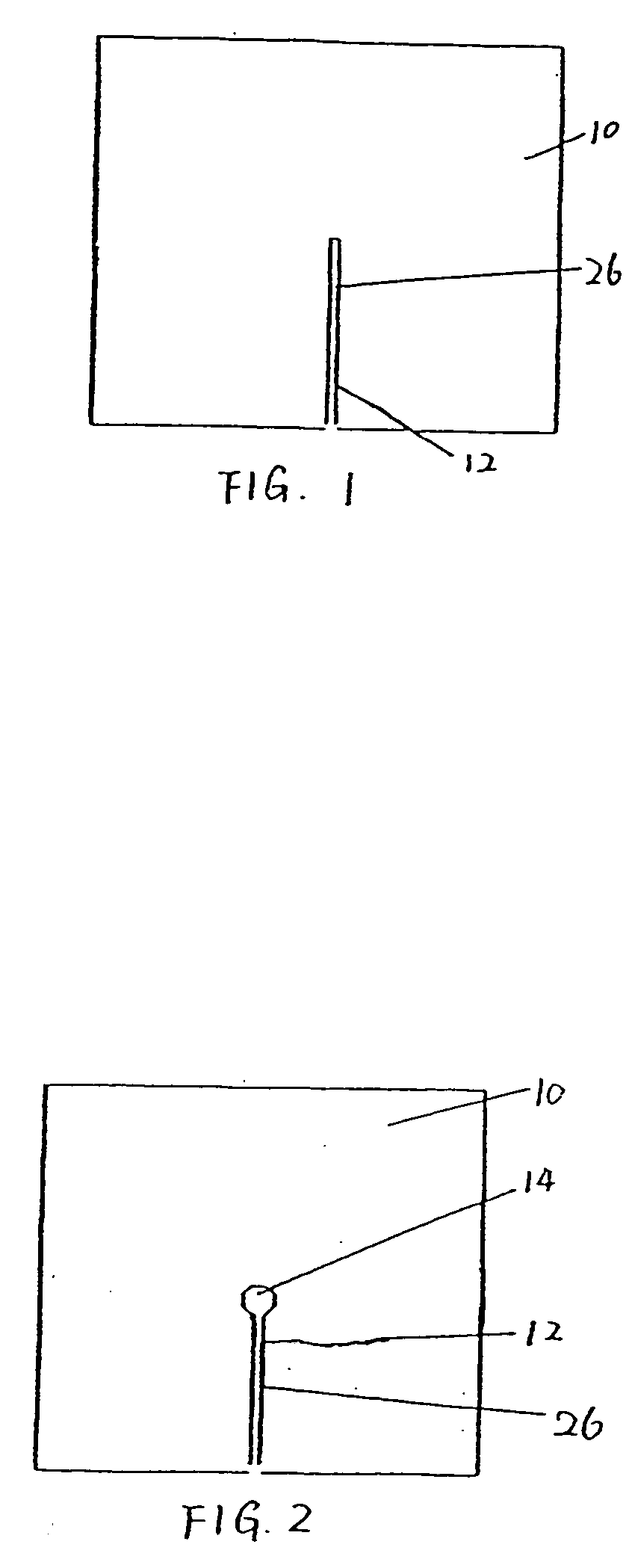Hemostasis pad and method
a technology of hemostasis pad and hematoma, which is applied in the field of hemostasis medical devices, can solve the problems of prolonging clotting time and complicated situation, and achieve the effect of reducing the time period required and reducing the likelihood of hematoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention is described hereinafter with specific references to the use of the present invention for sealing an incision or puncture wound with an indwelling tubular element, such as catheter, introducer or tube, through the incision or puncture wound. It is contemplated that the present invention may be used with any catheterization or other medical procedure such as laparoscopic or other minimally or less invasive surgeries wherein it is desirable to seal an incision or puncture wound in the patient to prevent the loss of the patient's body fluid therethrough.
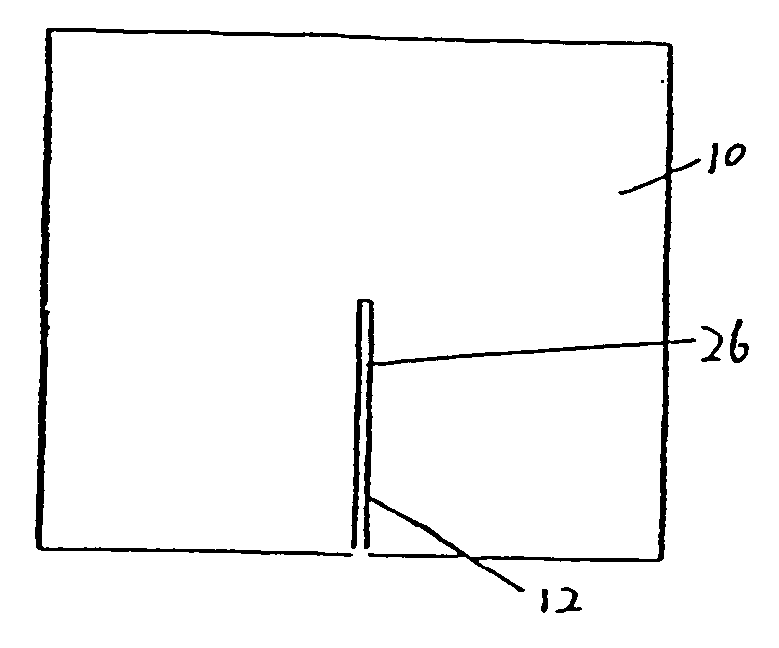
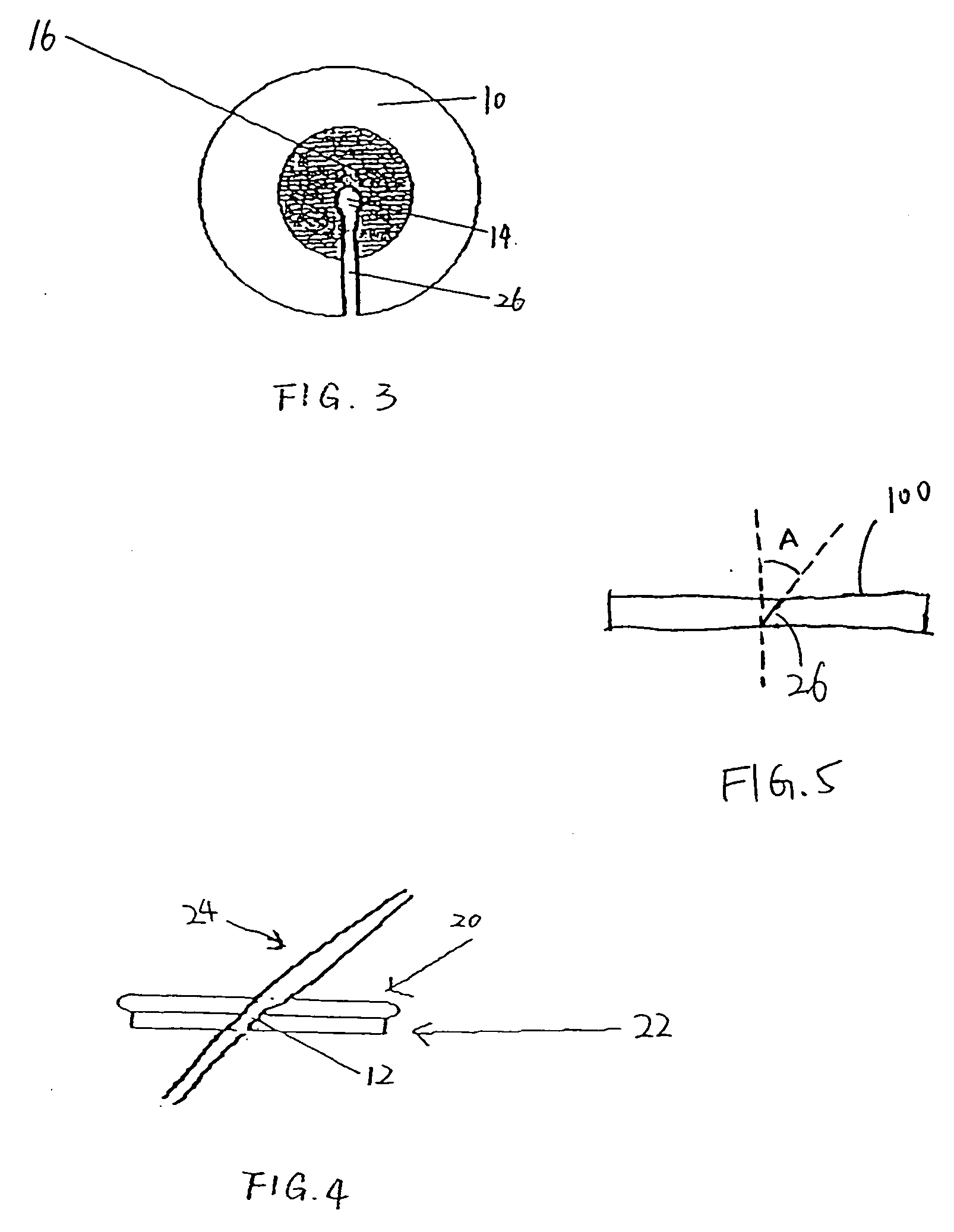
[0028]FIG. 1 is a top view of one preferred embodiment of the present inveniton. Referring to FIG. 1, a pad 10 defines an opening 12 through the pad 10. In a preferred embodiment, the opening is constructed as a slit or an elongated cut 26. As shown in FIG. 1, the slit 26 extends from an approximately central point to an edge of the pad 10. The slit is preferably sized to permit egress of an indwelling tubu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bias angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| bias angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com