Methods for generating high titer helper-free preparations of released recombinant AAV vectors
a technology of aav vector and aav plasmid, which is applied in the direction of hydrocarbon preparation catalysts, group 5/15 element organic compounds, peptides, etc., can solve the problems of inability to achieve ideal treatment, inability to produce vector byproducts, and inability to treat the subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Illustrative Production of Recombinant AAV Vector Using a Wild-Type Helper Virus (AD5) and a Temperature-Sensitive Helper Virus (AD TS149)
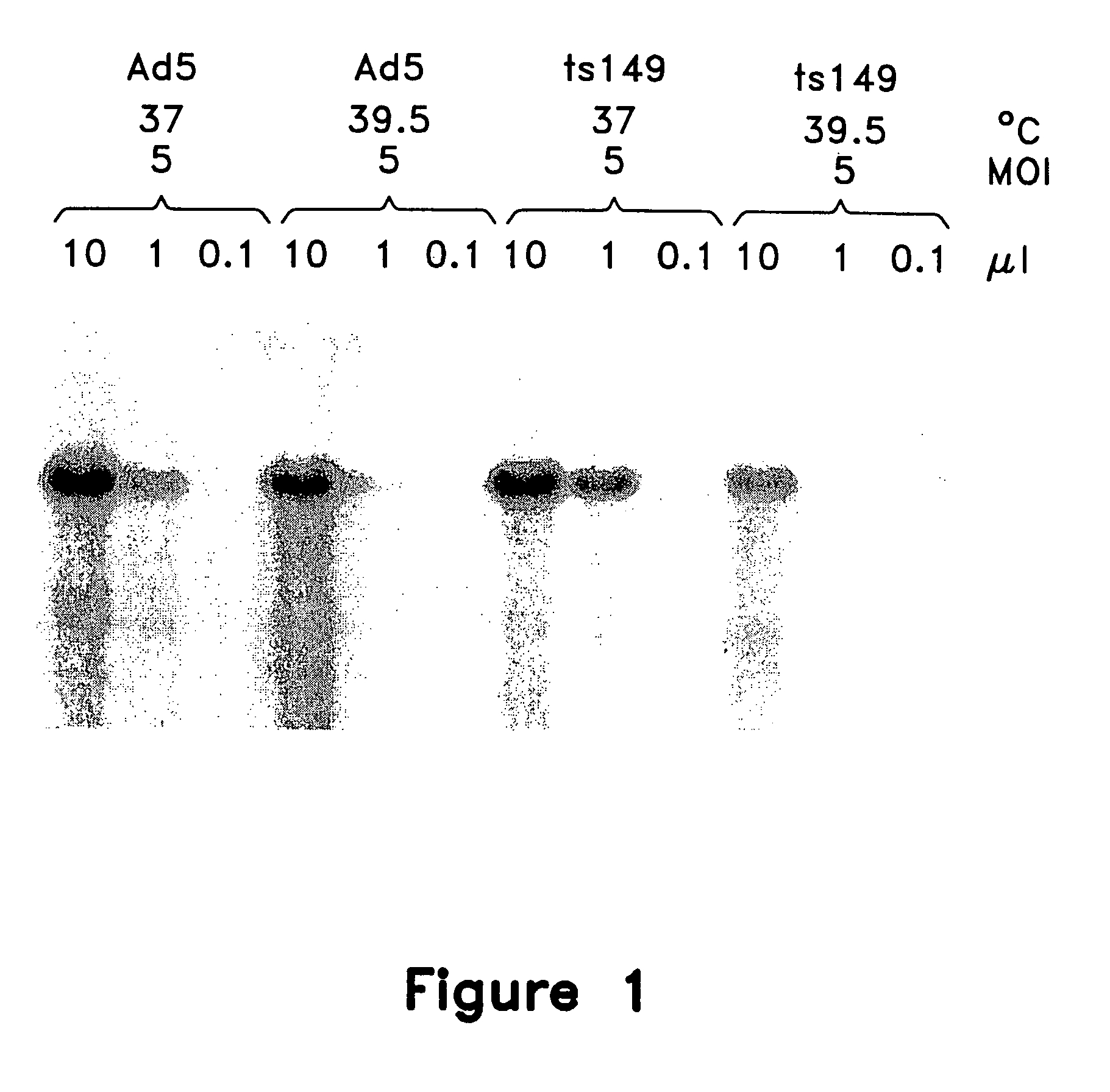
[0267] This example illustrates the use of a wild-type helper virus (Ad5) and a temperature-sensitive helper virus (Ad ts149) to provide helper functions for the replication of a recombinant AAV vector particle comprising a model therapeutic gene.
[0268] The ptgAAVCF plasmid consists of the left hand AAV2 ITR; a full length cystic fibrosis transmembrane regulator cDNA; a synthetic polyadenylation sequence based on the mouse β-globin polyadenylation sequence; AAV2 sequences downstream of the cap coding sequences; and the right-hand AAV2 ITR in a pBR322 plasmid backbone (Afione et al,, 1996). The pGEM-RS5 packaging plasmid was derived from the pHIVrep plasmid (Antoni et al., 1991) and consists of the U3 and R regions from the HIV-1 LTR; the rep and cap regions from AAV2 including the p19 and p40 promoters; pBR322 and pGEM plasmid sequences for bact...
example 2
Quantitation of RAAV and Adenovirus Titers in Vector Preparations
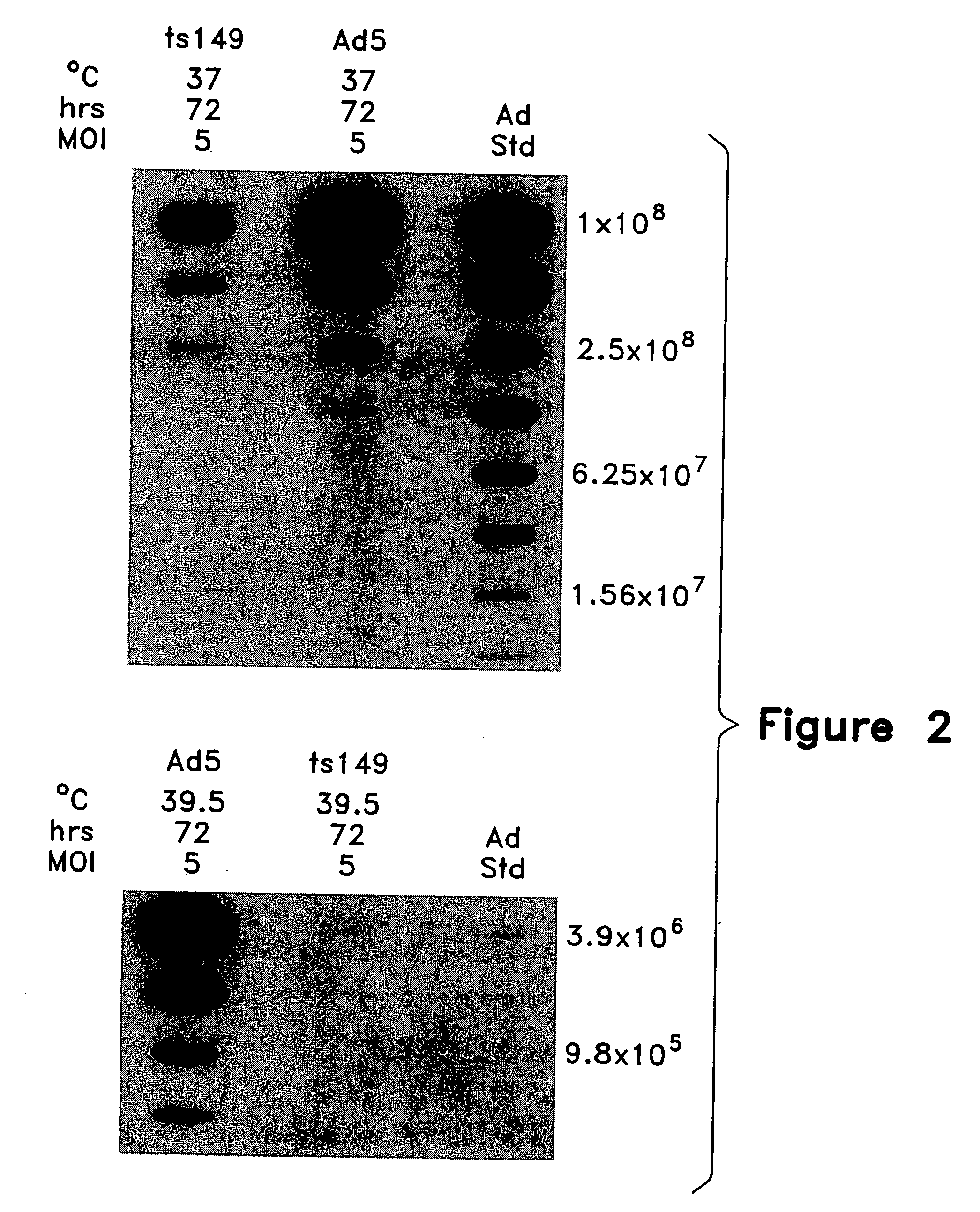
[0273] Cell lysates from the preceding example were assayed for production of rAAVCF vector by C37 replication assay and analyzed for adenovirus production by slot-blot hybridization.
[0274] HeLa C37 was constructed to allow inducible expression of AAV Rep proteins for rAAV vector replication. Briefly, an AAV Rep / Cap expression cassette consisting of the mouse metallothionein I promoter, AAV2 rep and cap genes and AAV transcription termination site was constructed. Also included in the plasmid was a neomycin resistance gene under the control of the SV40 early promoter, SV40 small T intron and the SV40 polyadenylation signal. HeLa cells were transfected with the plasmid and clones were selected in G418. A panel of clones was screened by a rAAV vector amplification assay. One clone, C37, demonstrated consistent and dose dependent amplification of rAAV vector following transduction and adenovirus infection.
[0275] Detect...
example 3
Optimization of Helper Function to Improve RAAV Production
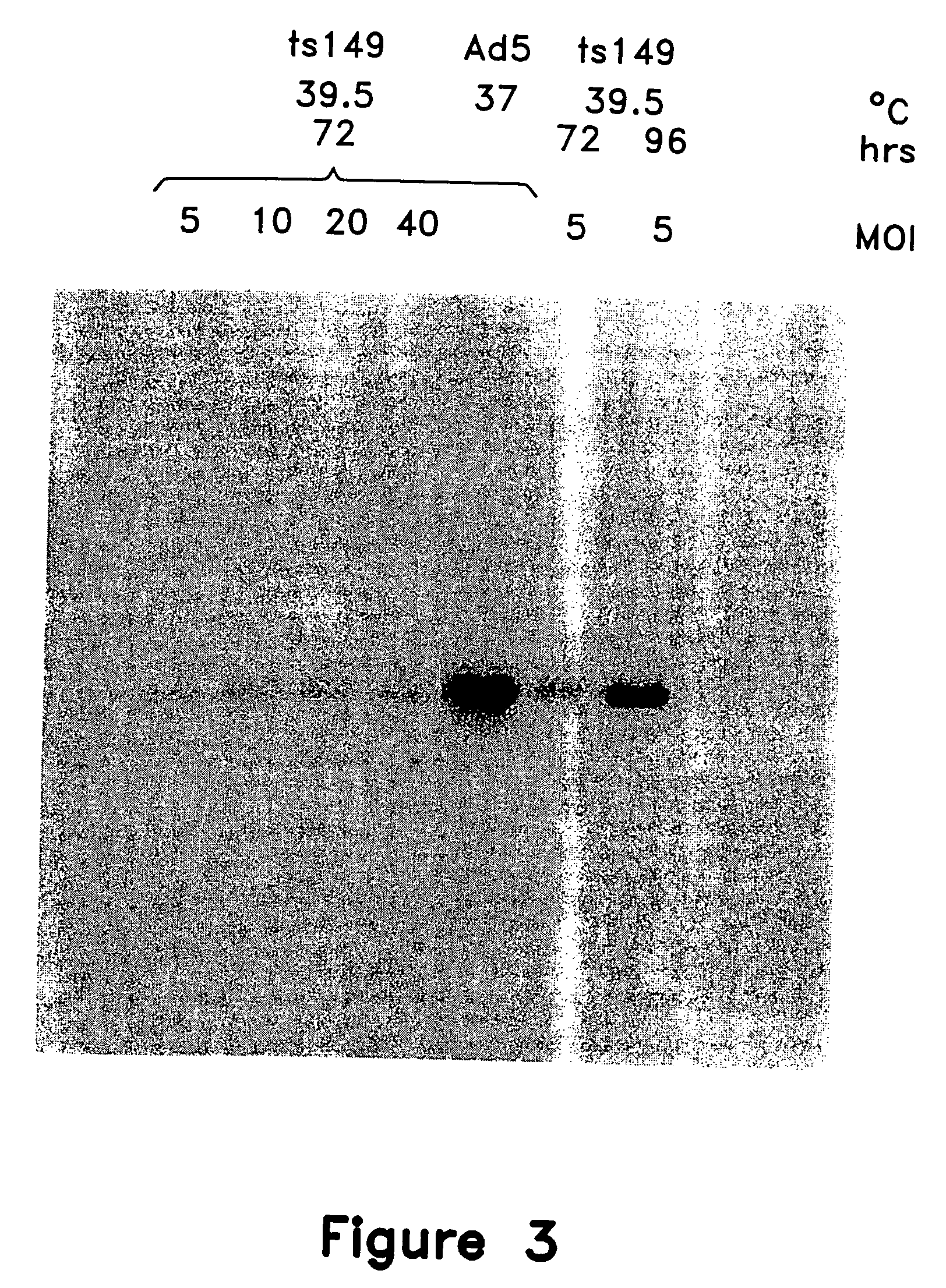
[0279] This example illustrates various attempts to improve the level of rAAV obtained when using temperature-sensitive helper virus. Increasing infection levels of the helper virus was unhelpful, but adjusting the kinetics was surprisingly effective.
[0280] The effects of increasing multiplicity of infection on vector production was evaluated first. 293-1 cells were infected with either Ad5 at a MOI of 5 or ts149 at various MOI, followed by transient co-transfection with vector and packaging plasmids. After 72 hours, the cells were lysed and assayed for production of rAAVCF vector by C37 vector replication assay and analyzed for adenovirus production by slot-blot hybridization. An additional 96 hour time point was collected for cells infected with ts149 at a MOI of 5.
[0281]FIG. 3 shows the results of the rAAVCF replication assay conducted on cell lysates prepared with ts149 at various MOI. Increasing the MOI of ts149 did n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| permissive temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com