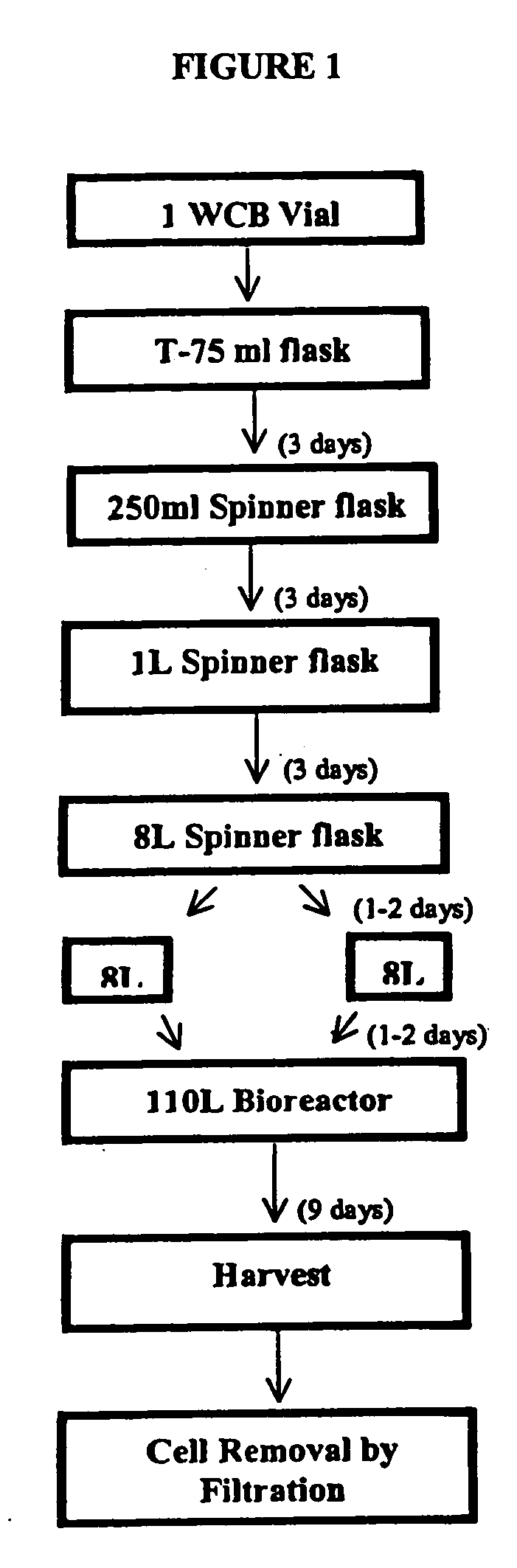
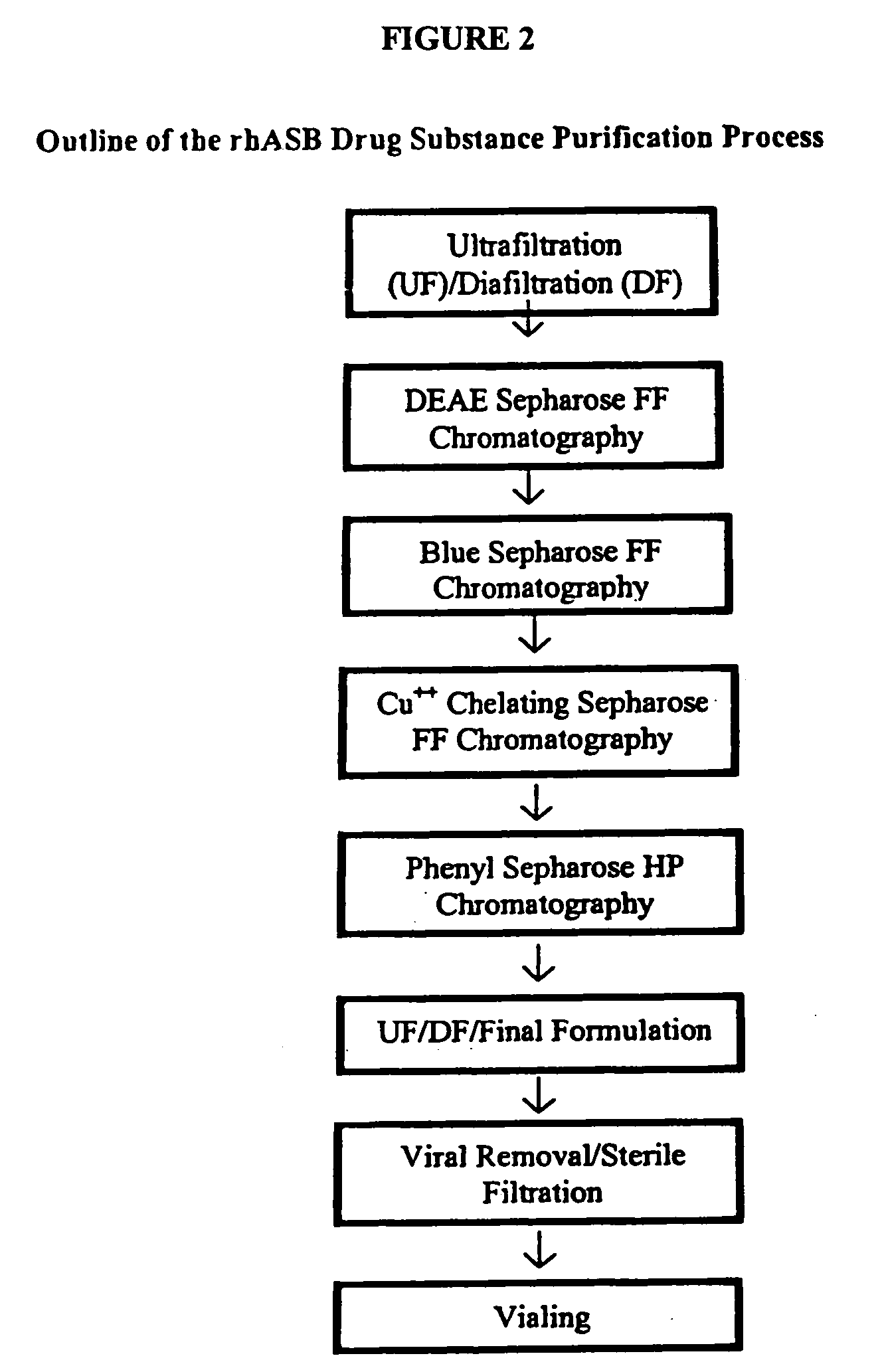
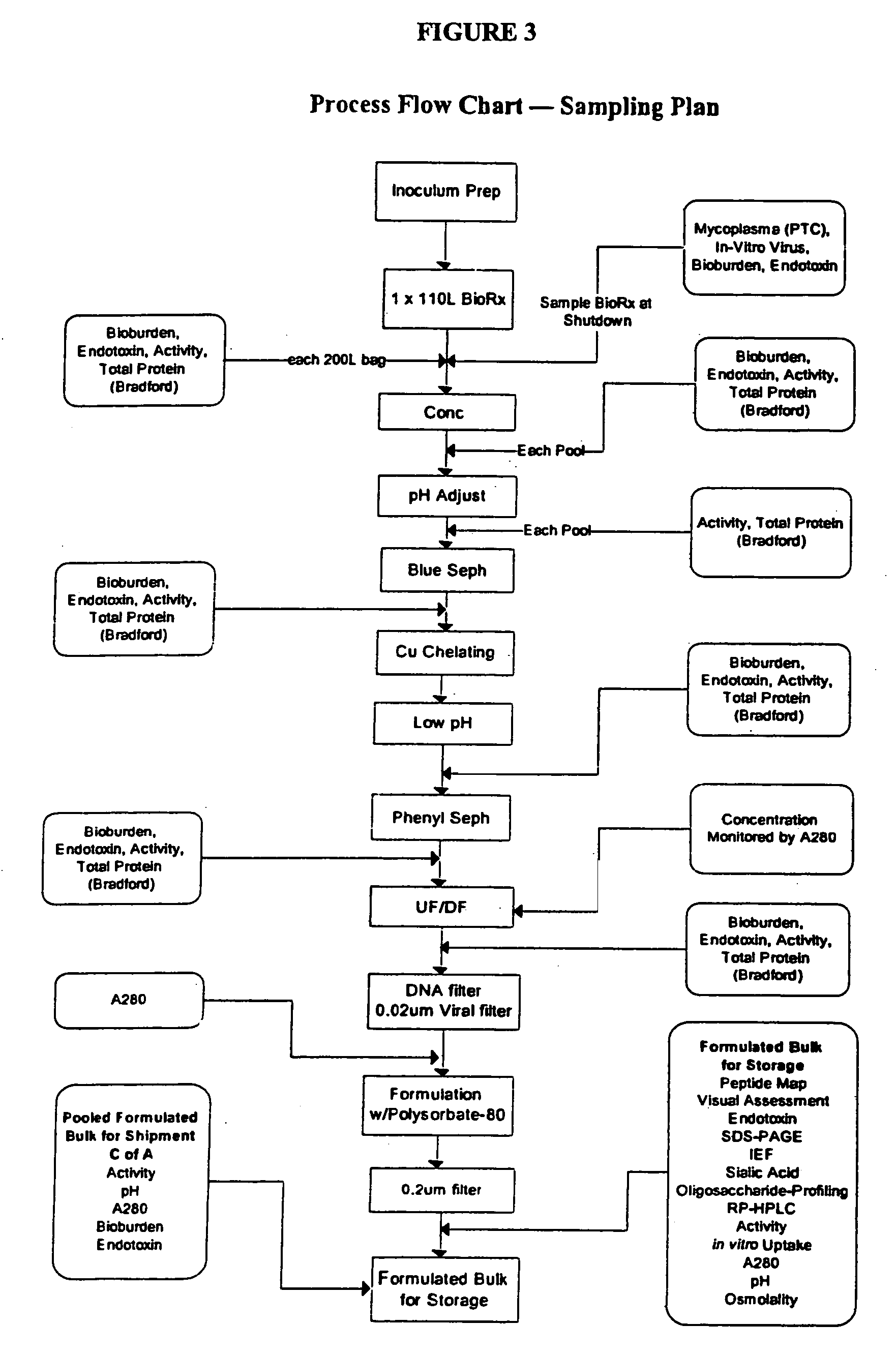
Precursor N-acetylgalactosamine-4 sulfatase, methods of treatment using said enzyme and methods for producing and purifying said enzyme
a technology of n-acetylgalactosamine and sulfatase, which is applied in the fields of biochemistry and molecular biology, can solve the problems of affecting learning and development, no acceptable clinical dosage or medical formulation, and substantial morbidity and mortality, and achieves high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Evaluation with Recombinant Human N-acetylgalactosamine-4-sulfatase
Summary
[0095] The indication for recombinant human N-acetylgalactosamine-4-sulfatase (rhASB) is the treatment of MPS VI, also known as Maroteaux-Lamy Syndrome. We propose a clinical development program for rhASB consisting of an initial open-label clinical trial that will provide an assessment of weekly infusions of the enzyme for safety, pharmacokinetics, and initial response of both surrogate and defined clinical endpoints. The trial will be conducted for a minimum of three months to collect sufficient safety information for 5 evaluable patients. At this time, should the initial dose of 1 mg / kg not produce a reasonable reduction in excess urinary glycosaminoglycans or produce a significant direct clinical benefit, the dose will be doubled and maintained for an additional three months to establish safety and to evaluate further efficacy.
Objectives
[0096] Our primary objective is to demonstrate safety o...
example 2
[0105] A comprehensive review of the available information for the MPS VI cat and relevant pharmacology and toxicology studies is presented below: Enzyme replacement therapy has been established as a promising treatment for a variety of inherited metabolic disorders such as Gaucher Disease, Fabry Disease and Mucopolysaccharidosis I. In some of these disorders a natural animal model offers the ability to predict the clinical efficacy of human treatment during pre-clinical studies. This was found to be true in MPS I (canine model). With this in mind, studies have been performed with the MPS VI cat prior to the commencement of human studies for this disease. Sufficient safety and efficacy data exist to proceed with a clinical trial in human MPS VI patients.
[0106] Studies of rhASB MPS VI cats indicate that no cat has died as a result of drug administration. As predicted, experiments in MPS VI cats also indicate that rhASB uptake is dependent on the presence of mannose 6-phosphate modif...
example 3
Distribution and Feasibility
[0112] An initial study was performed to document enzyme uptake and distribution, and to serve as a pilot study of potential endpoints for future efficacy studies (Crawley, et al., J. Clin. Invest. 97.1864-1873 (1996)). Recombinant human ASB was administered by bolus injection to affected cats once per week or once every two weeks at 0.5 up to 1.5 mg / kg. Evaluation of one untreated MPS VI cat (Cat D), and one normal cat provided the values from which comparisons were drawn. The data from the one untreated cat was further supported by historical assessment of 38 additional untreated cats. The acute uptake and distribution studies were conducted in normal cats using an immune assay technique that allowed the detection of human ASB in the presence of normal cat enzyme.
[0113] The major conclusions of these studies demonstrated wide uptake of enzyme with the expected predominance of liver and spleen uptake as observed in other enzyme replacement studies in M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com