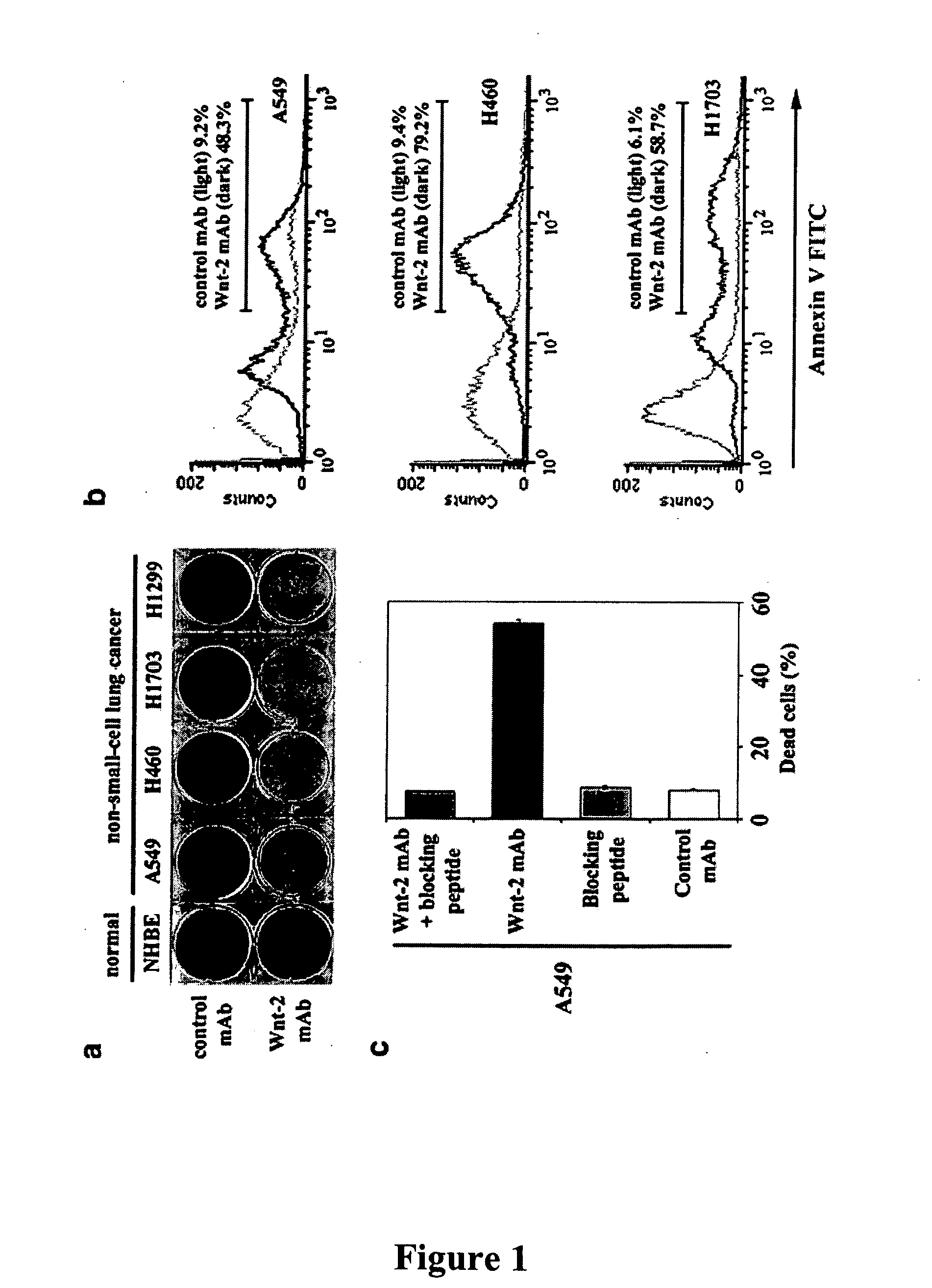
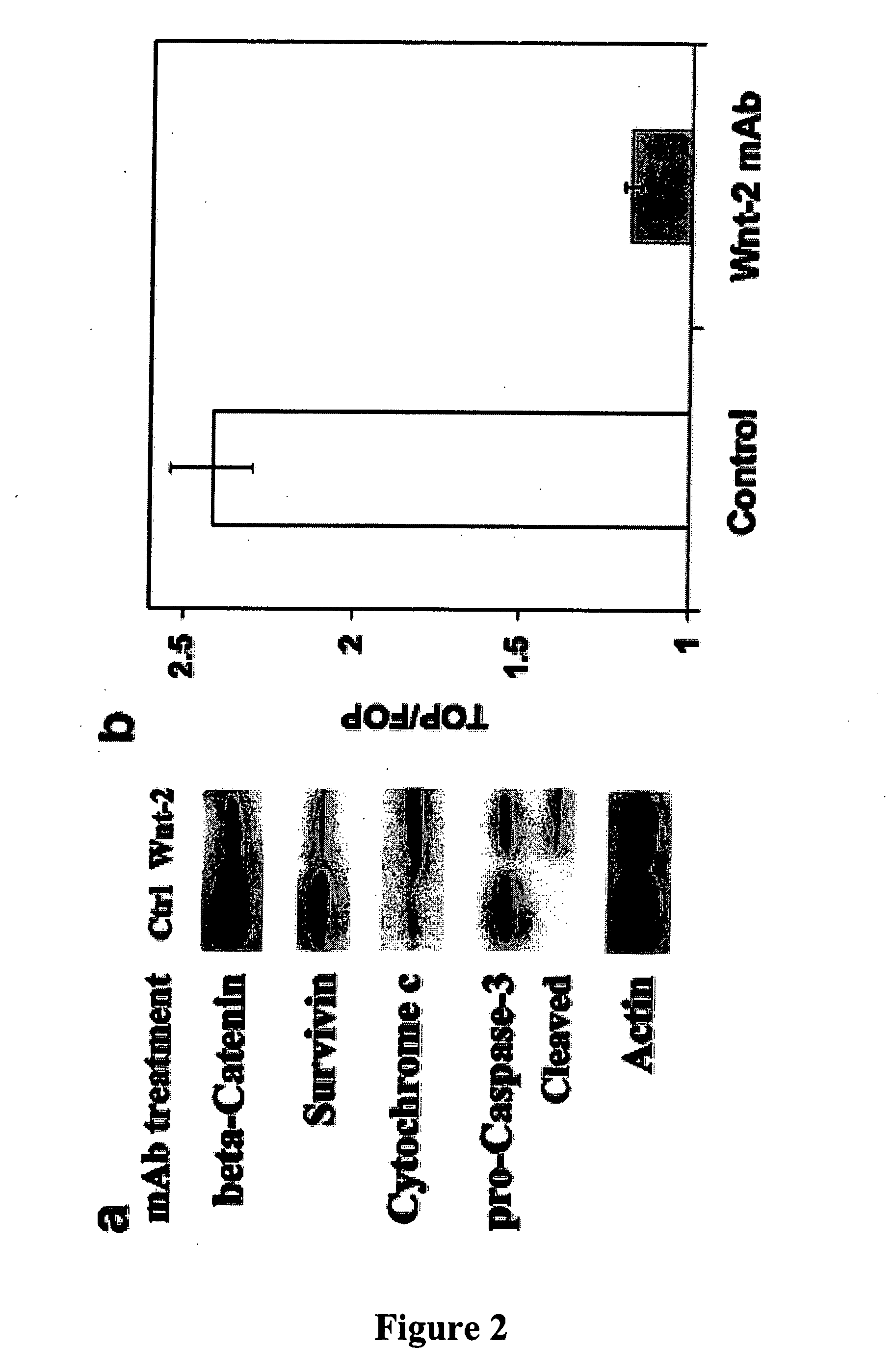
Methods for treating cancer using anti-Wnt2 monoclonal antibodies and siRNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0221] 1. Cell Lines and Tissue Samples
[0222] Human non-small-cell lung cancer (NSCLC) cell lines (A549, NCI-H1703, H460, and H1299), mesothelioma cancer cell lines (NCI-H2052, H28 and H513), melanoma cell lines (LOX, FEM, FEMX, and SK-Mel-2), breast cancer cell lines (MCF-7 and HuL100, and colon cancer cell lines (HCT116 and SW480) were from American Type Culture Collections (ATCC, Manassas, Va.). Mesothelioma cancer cell lines were obtained from the following sources: LRK1A and REN through a generous gift from Dr. Steven Albelda (University of Pennsylvania, Philadelphia, Pa.), NCI-H2052, H28 and H513 from American Type Culture Collections (ATCC, Manassas, Va.), MS-1 and NCI-H290 from NIH (Frederick, Md.) and LP9 were from the Cell Culture Core Facility at Harvard University (Boston, Mass.). All cell lines except LP9 were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (100 IU / ml) and streptomycin (100 μg / ml). LP9 was cultured in M1...
example 2
Identification of Antigenic Wnt2 Peptides
[0243] Antigenic peptides of human Wnt2 protein were determined using various methods. For example, the EMBOSS (Parker et al., Biochemistry 25:5425-5432 (1986)) finds antigenic sites in proteins. Antigenic peptides were also determined using the method of Kolaskar and Tangaonkar (K&T; FEBS Lett. (1990) 276(1-2):172-4). Both methods led to the identification of similar antigenic peptides of human Wnt2 (Table 1). While most of the antigenic peptide sequences identified can be used to generate antibodies that specifically bind to human Wnt2, some antibodies may also bind to other Wnt proteins due to amino acid sequence homology among various Wnt proteins with human Wnt2. For example, the amino acid sequences of SEQ ID NO:42 and SEQ ID NO:44 have homology to human Wnt2B (Wnt13); the amino acid sequence of SEQ ID NO:43 has homology to human Wnt2B (Wnt13), Wnt3, Wnt3A, Wnt5B, and Wnt10A; the amino acid sequence of SEQ ID NO:45 has homology to huma...
example 3
Generation of Anti-Wnt2 Monoclonal Antibodies and Antibody Incubation with Cells
[0244] The antigen used to raise monoclonal anti-Wnt2 antibodies was a synthetic peptide corresponding to amino acid residues 49-63 of the human Wnt2 (Ac-SSQRQLCHRHPDVMR-amide, SEQ ID NO:2). This antigen was chosen bioinformatically based on its hydrophilicity (Parker et al., (1986) Biochemistry 25(19):5425-32), antigenicity (Welling et al., (1985) FEBS Lett. 188(2):215-8), accessibility (Janin, Nature 277:491-2 (1979)), sequence homology (BLAST search), and N-terminal vicinity.
[0245] The anti-Wnt2 mouse monoclonal antibody (IgG1) was custom-made at Rockland Inc. (Gilbertsville, Pa.). Several hybridoma cell lines were generated of which five were characterized in detail: (1) 17F7.G7 (Subclone A; FIG. 14); (2) 17F7.G7 (Subclone B; FIG. 18); (3) 17F7.E5 (FIG. 16); (4) 8B11.D2 (FIG. 15); and (5) 8B11.H6 (FIG. 17). Two different light chains were found in 8B11.H6, termed 8B11.H6 (Chain1) and 8B11.H6 (Chain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com