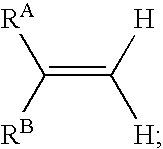
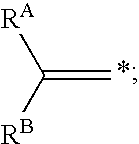
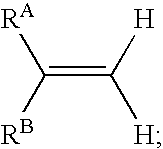
Reagents and methods for labeling terminal olefins
a technology of terminal olefins and reagents, applied in the field of reagents and methods for labeling terminal olefins, can solve the problems of limited practical use of biosynthetic labeling with tritium, limited practical application, and non-specific isotopic substitution could present a serious problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
C-19′ Deuteration of Halichondrin Analog ER-810951
[0148]
[0149] ER-810951 (1 wt, 1 eq) and Grubb's 2nd generation olefin metathesis catalyst (1.67 wt, 1.5 eq) were placed in a 25 mL Schlenk-type vessel. The atmosphere was exchanged for nitrogen gas three times, then evacuated. Ethylene-d4 (25 mL, 800 eq.) was charged into the evacuated vessel. Dichloromethane 92 mL was charged into the vessel. The vessel was sealed and placed in a 35° C. bath. The mixture was stirred for 18 hours. The reaction mixture was cooled to room temperature, and the vessel was opened. The mixture was purified by flash chromatography. The product was concentrated to give a white-brown solid (0.3 wt, 0.3 eq.). Analysis of the product by 1H NMR (CD3OD), d1=3 sec showed incorporation at the C19 methylene approaching 75%. MS analysis (M+Na, 954) showed significant enrichment of M+2, also approaching 75-80%.
example 2
C-19′ Carbon-14 Labeling of Halichondrin Analog ER-813018
[0150]
[0151] A reaction flask was charged with ER-813018 (1 wt, 1 equiv.).* Toluene (100 vol) was added. The solution was frozen with liquid nitrogen, vacuum was applied, and the solution was thawed. The reaction mixture was re-frozen with liquid nitrogen, and 1,2-14C-ethylene (2-3 equiv.) was transferred to the reaction vessel. The vessel was sealed and warmed to room temperature. The reaction mixture was heated to 60-65° C. After the desired temperature was reached, a solution of Grubbs 2nd generation catalyst (5 mol %) in toluene was added to the reaction mixture. The resulting mixture was stirred for 20-60 minutes. The reaction was cooled.** The reaction mixture was sampled and analyzed by mass spectrometry versus an unlabeled standard to determine the specific activity. The above process was repeated until the desired level of incorporation was achieved.***, ****
[0152] * The reaction flask size was determined such that ...
example 3
C-19′ Carbon-14 Labeling of Halichondrin Analogs ER-813016 and ER-813020
[0169] C-19′13C labeling was carried out using procedure similar to that described in Example 2 with the following substrates:
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com