Biopsy localization method and device
a biopsy and localization technology, applied in the field of biopsy localization methods and devices, can solve the problems that surgeons often have difficulty in determining the precise relationship of previously excised tissue to the surgical wound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
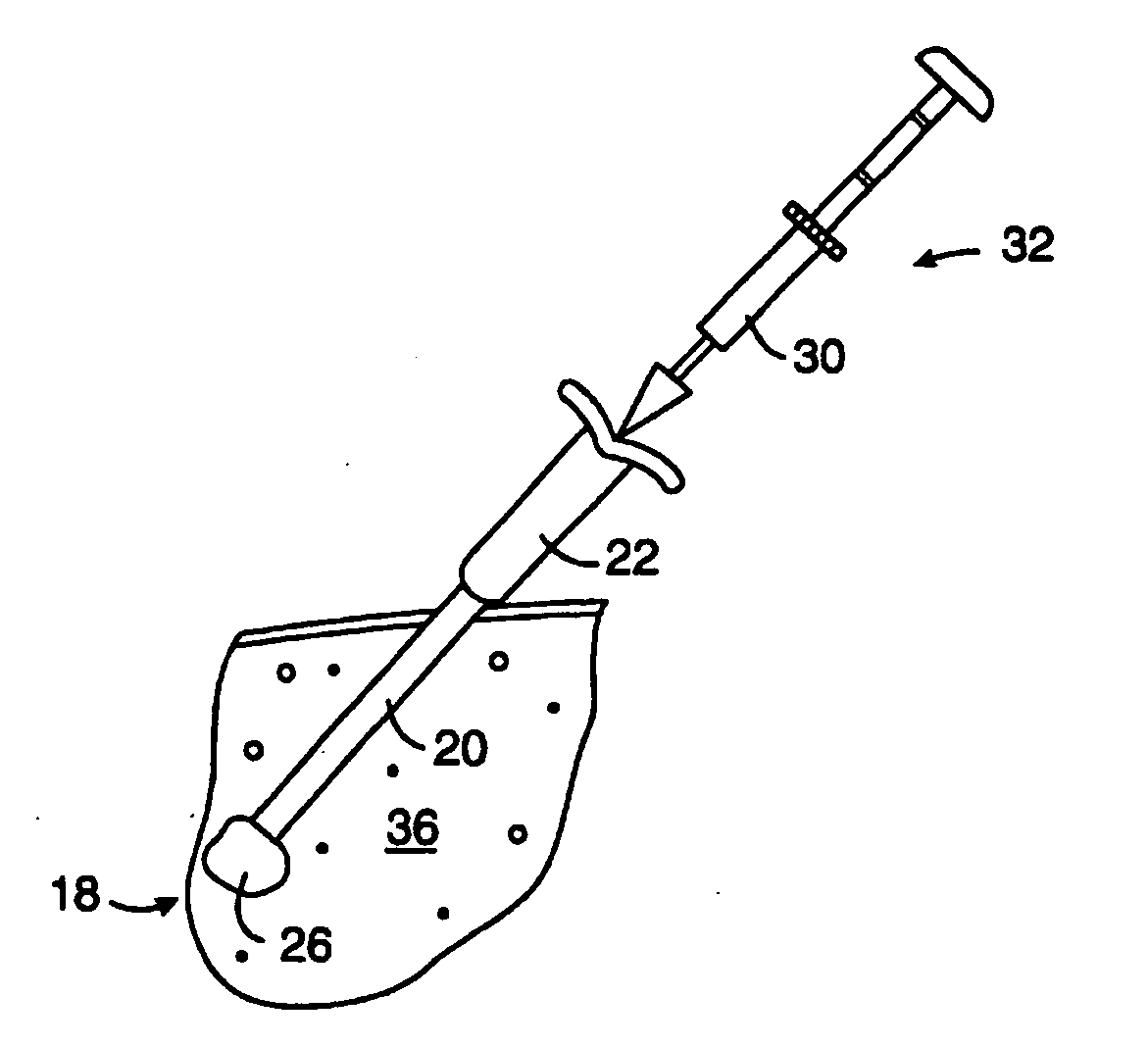
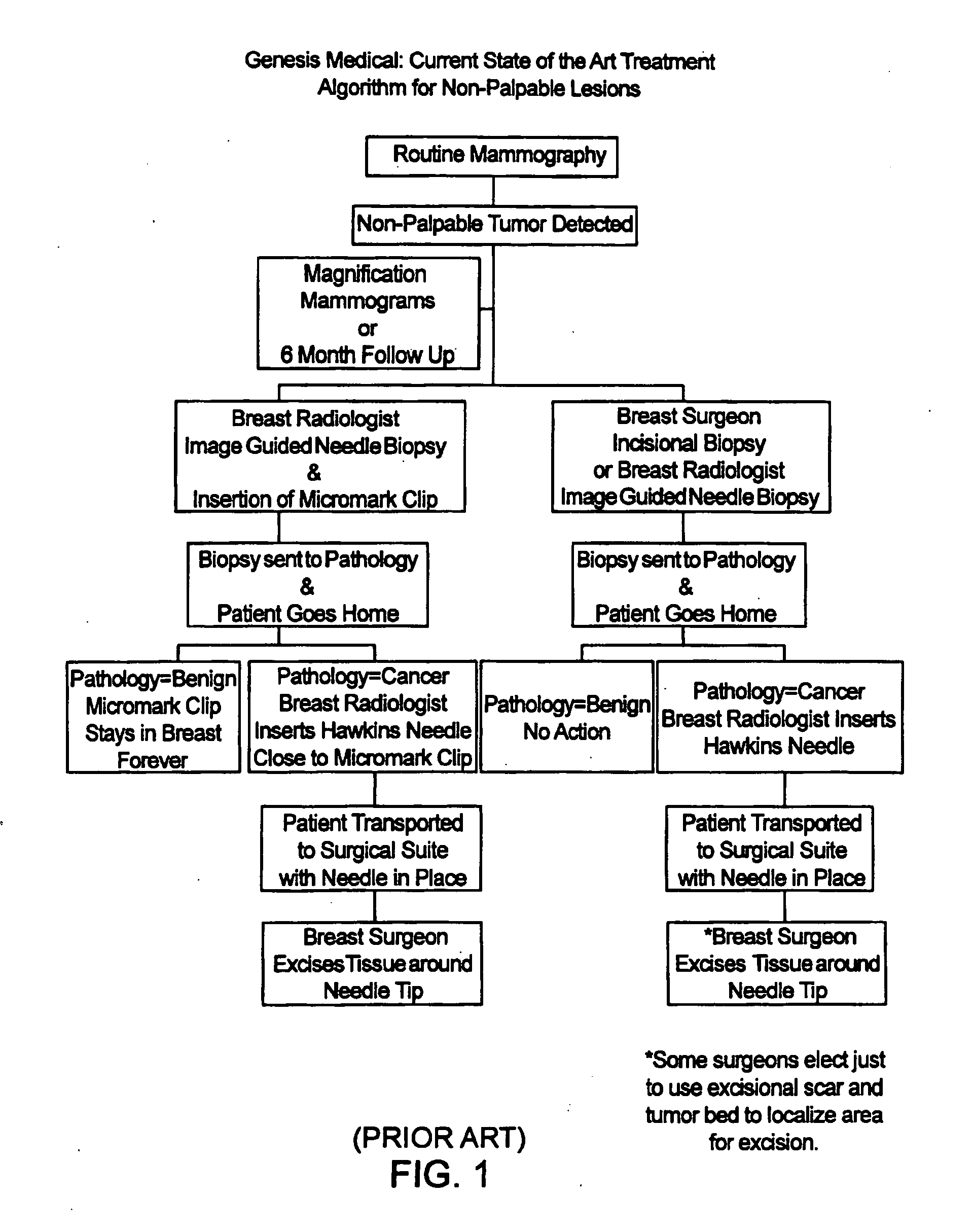
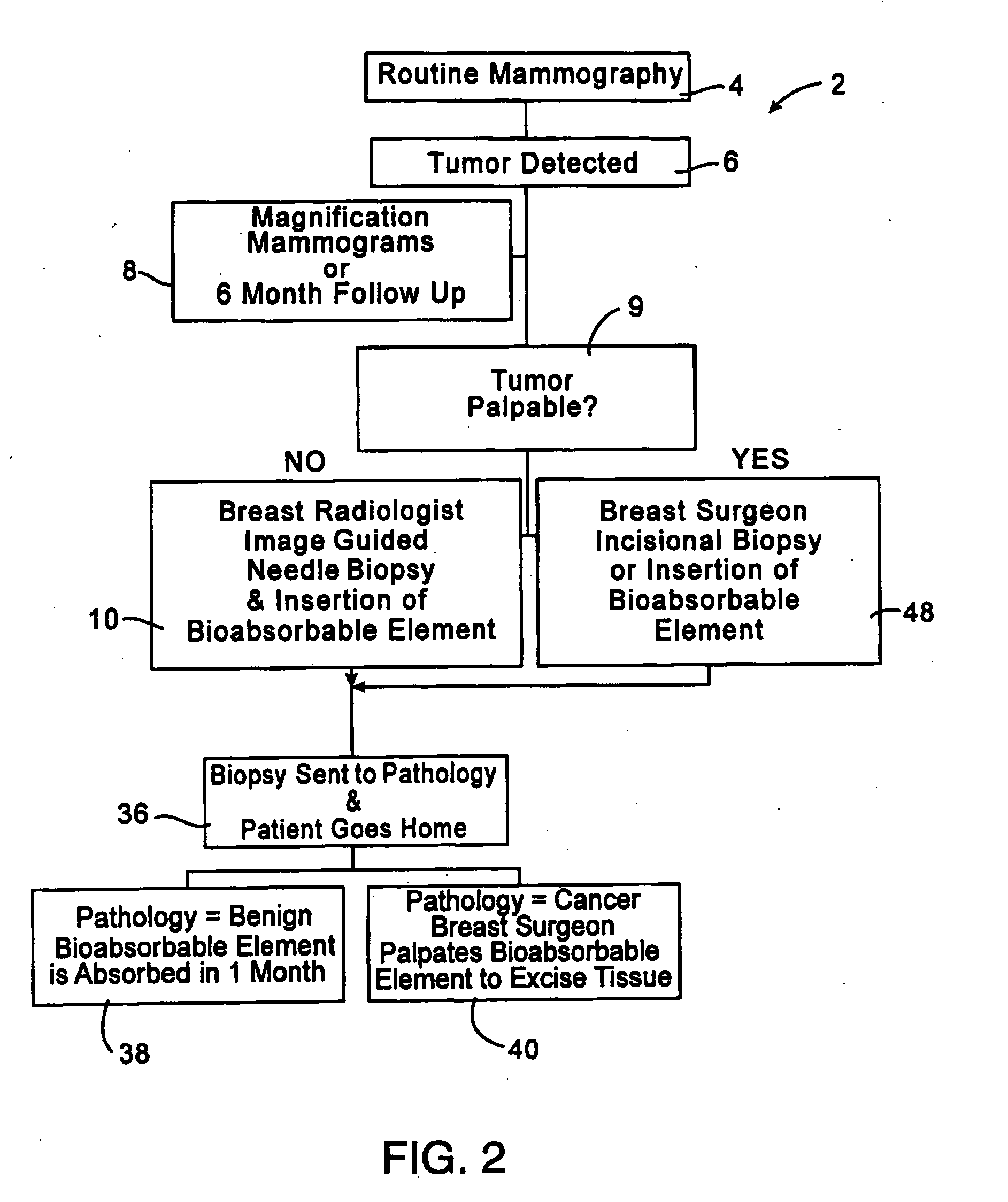
[0023]FIG. 2 illustrates a treatment algorithm 2 according to the present invention. As a result of a routine mammography 4, a tumor or other abnormality may be detected as at 6. The typical response will often include additional magnification mammograms or a follow-up mammogram scheduled for some time in the future, such as six months. This is indicated at 8. If the tumor is not palpable, see 9, an image guided needle biopsy by a breast radiologist is typically conducted as at 10. Image guided needle biopsies can be done in a number of ways. Presently, stereotactic (x-ray) and ultrasound guided needle biopsies are commonly used, primarily because of their accuracy, speed and minimal trauma to the patient. Stereotactic needle biopsies typically use a stereotactic table, such as made by Fisher or Lorad, which provides mammography (x-ray) guidance to a biopsy needle assembly. Ultrasound guided biopsies can be conducted with any one of a number of commercially available instruments. An...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com