Polyol-based method for producing ultra-fine nickel powders
a technology of nickel powder and polyol, which is applied in the direction of metal-working apparatus, transportation and packaging, etc., can solve the problems of large quantities of organic solvents per unit weight of metallic powder produced, low concentration of metallic precursors, and wide size distribution of metallic powder produced using these procedures, and achieves low agglomeration, high crystallinity and oxidation resistance, and tight size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials
[0048] The copper carbonate (CuCO3) was supplied by Shepherd Chemical Co. 1,2-PG and DEG were obtained from Alfa Aesar (Ward Hill, Mass.). 1,3-PG and PE were obtained from Avocado Research Chemical Ltd., while TEA was purchased from Aldrich (Milwaukee, Wis.).
example 2
Copper Particles Synthesis
[0049] All experiments were carried out in a 1 L-4-necked round flask equipped with a Dean Stark trap and a refluxing condenser. The stirring was provided by a two inch Teflon—blade connected to a variable speed mixer. The amount of cupric carbonate used in the precipitation process was in general kept at 200 g (1.62 mol) although smaller or larger amounts were occasionally used as well (i.e., 87 g and 300 g). The CuCO3 was added into 500 cm3 polyols or polyols mixture containing 15 g PE (for 200 g CuCO3). The dispersant agent (PE) was initially added in polyols and heated at low power (10%) in the heating mantle to bring the temperature to 70° C. The required amounts of CuCO3 were added into the flask at 80-85° C. after the PE was fully dissolved. The CuCO3 / polyol mixture was stirred at 500 RPM in all experiments. The mixture was then heated at 50% setting of heating power until the temperature reaches 180-185° C. The copper particles obtained were washed...
example 3
Particles Characterization
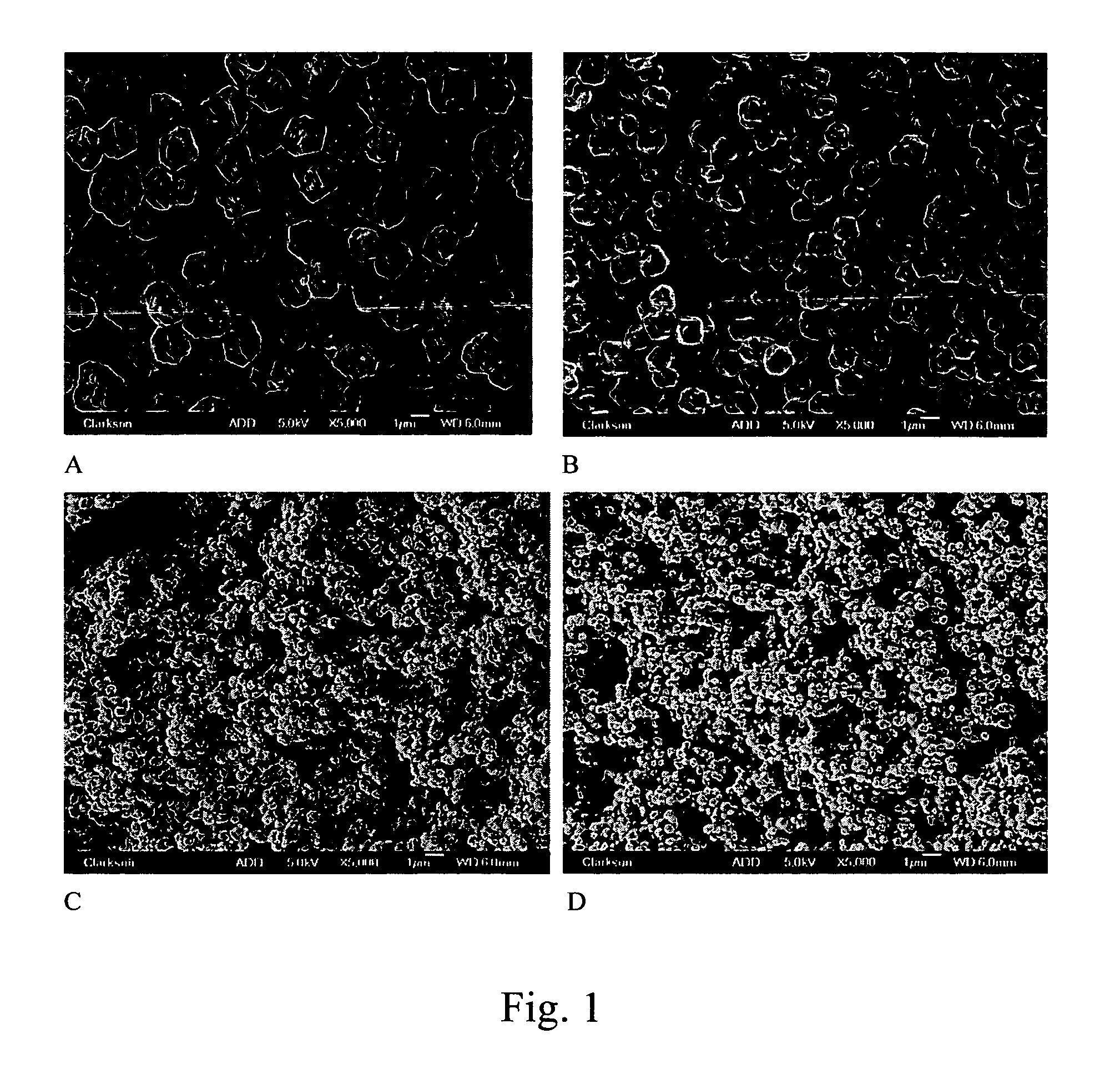
[0050] The morphology of copper particles was investigated by scanning electron microscopy (SEM) using a JEOL-JSM 6300 scanning microscope at 15 kV accelerating voltage and the magnification between 2500 and 10000. Also, the copper powders were analyzed by field emission scanning electron microscopy (FE-SEM) with 5 kV accelerating voltage and the same range of magnification using a JEOL JSM-7400F field emission scanning electron microscope.
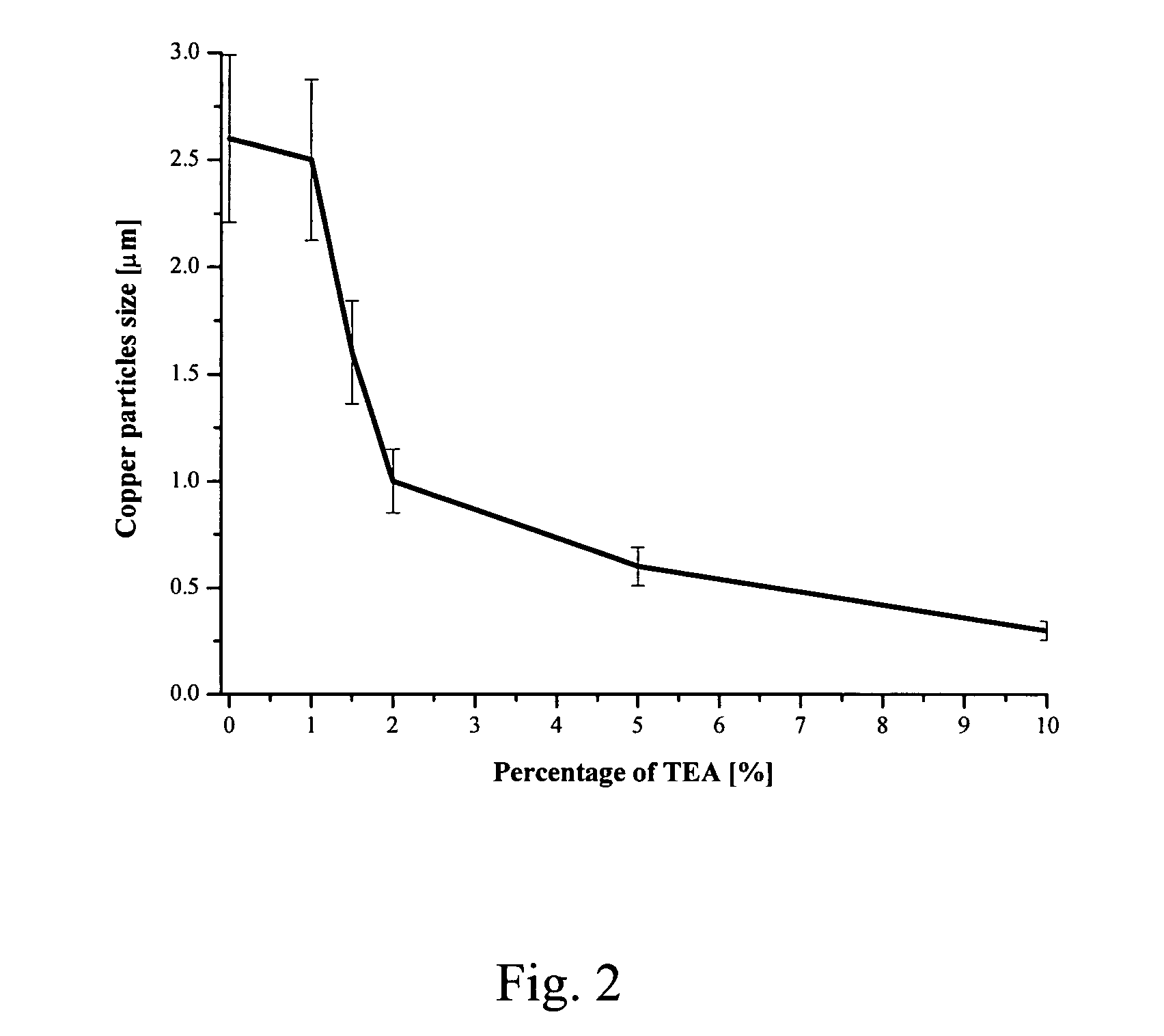
[0051] Discussed below are results obtained by the inventors in connection with the experiments of Example 1-3:
[0052] In order to evaluate the effect of pH in the formation of Cu particles in polyols, variable amounts of triethanolamine (TEA) were added into the dispersion of CuCO3 prior to the heating as shown in Table 1. The reaction time in the presence of TEA varied between 3 and 4 hours, the addition of more base tending to speed up the reaction. The images of copper particles produced in the manner described in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com