Pharmaceutical compositions
a composition and pharmaceutical technology, applied in the field of oral pharmaceutical compositions, can solve the problems of limited utility as a pharmaceutical, low and variable bioavailability, and difficulty in formulating stable galenic compositions, and achieve the effects of convenient administration, stable, and high bioavailability of drug substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
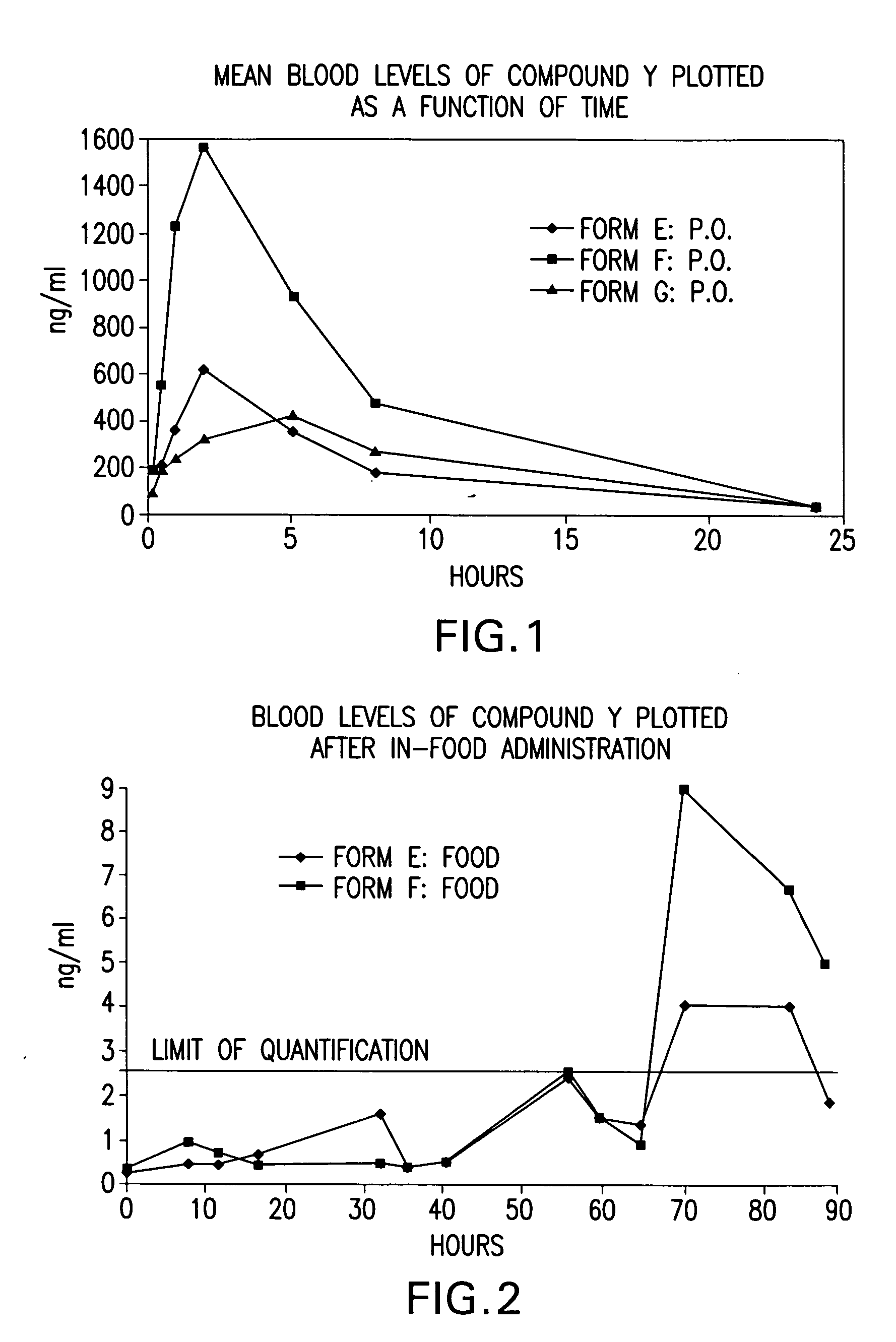
Image
Examples
example 2
[0084] A solid dispersion composition is prepared containing the following ingredients (in parts by weight):
Compound X16.7HPMC 3 cps66.7Poloxamer 188 (from BASF)16.7
The composition (Form B) is prepared in analogous manner to that in Example 1.
example 3
[0085] A solid dispersion composition is prepared containing the following ingredients (in parts by weight):
Compound X16.7HPMC 3 cps66.7TPGS*16.7
*tocopherol polyethylene glycol succinate
The composition (Form C) is prepared in analogous manner to that in Example 1.
example 4
[0086] A solid dispersion composition is prepared containing the following ingredients (in parts by weight):
Compound X10HPMC 3 cps80Solulan C24 (from Amerchol)10
The composition (Form D) is prepared in analogous manner to that in Example 1.
[0087] The above compositions Forms A to D may be formed into tablets, filled into capsules, or powdered and packaged in sachets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com