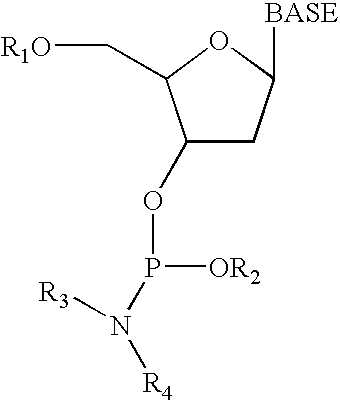
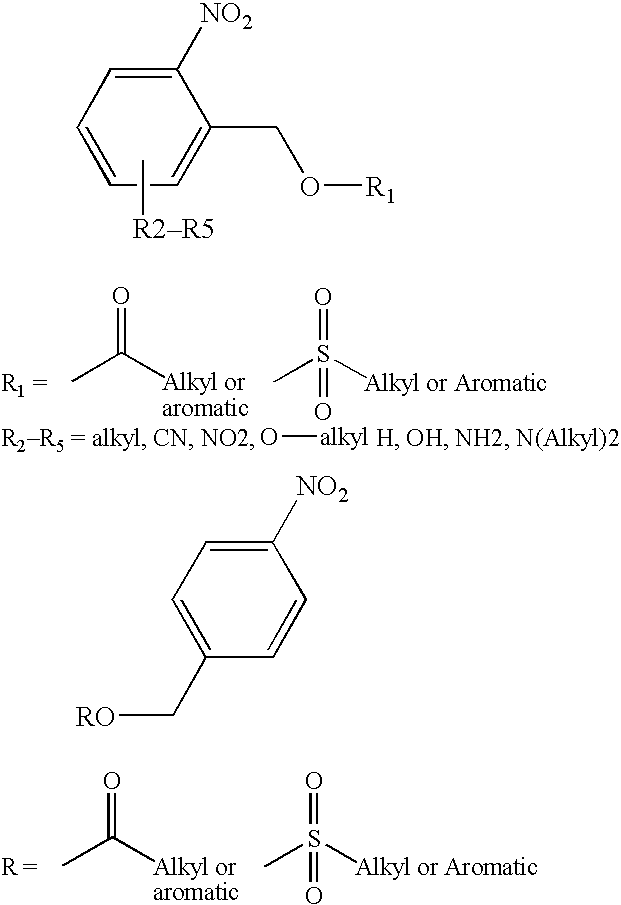
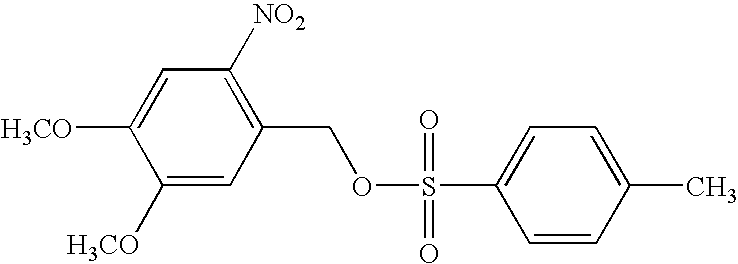
Chemical amplification for the synthesis of patterned arrays
a technology of patterned arrays and synthesis methods, applied in bulk chemical production, nuclear tetramethylamine libraries, biomass after-treatment, etc., can solve the problems of geysen et al., which is limited to producing 96 different polymers on pins spaced in the dimensions of a standard microliter plate, and achieves high synthesis fidelity, small synthesis feature, and improved synthesis resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Removal of Protecting Groups by Acid Amplification
[0086] Efficient removal of protective groups as taught by the present invention is demonstrated in the following experiment.
[0087] A system using an ester of toluenesulfonic acid as a PAAC and an autocatalytic ester of pentafluorobenzoic acid (1,4-cyclohex-2-enediylbis-(pentafluorobenzoate)) as an enhancer was employed. An experiment was conducted to determine time and intensity required to achieve efficient deprotection.
[0088] The synthesis of 1,4-cyclohex-2-enediylbis-(pentafluorobenzoate) and 2-nitro-3,4-dimethoxybenzyl tosylate were carried out according to Houlihan et al., Chemistry of Mat. 3:462-471, 1991. The yields were 54% and 62%, respectively.
[0089] Solutions containing poly (methyl methacrylate) (PMMA, average molecular weight of 15,000 dalton) (14.0 wt %), 1,4-cyclohex-2-enediylbis-(pentafluorobenzoate) (7.0 wt %), and 2-nitro-3,4-dimethoxybenzyl tosylate (0.5, 0.8, 1.2, 1.6, or 2.3 wt %) in cyclohexanone were spin ...
example ii
High Resolution Synthesis of Polynucleotide and Hybridization with an Polynucleotide Probe
[0094] Another important consideration for applying the techniques disclosed herein is whether the deprotection procedure interferes with the subsequent synthesis and functioning of the desired polymer arrays. The following experiment shows that functional polynucleotide arrays were synthesized by the method of the current invention.
[0095] A combination of a PAC and an enhancer in the form of a masked acid was used to synthesize a standard checkerboard pattern of an polynucleotide on a glass slide. The resulting glass slide containing polynucleotide arrays was hybridized to a complementary polynucleotide probe to test resolution and integrity of the arrays.
[0096] Solutions containing poly (methyl methacrylate) (PMMA, average molecular weight 15,000) (14.0 wt %), 1,4-cyclohex-2-enediyl-bis(pentafluorobenzoate) (7.0 wt %), and 2-nitro-3,4-dimethoxybenzyl tosylate (1, 2 wt %) in cyclohexanone w...
example iii
Lithographic Evaluation
[0103] As shown in FIG. 4, the high contrast observed in photo processes reflects the nonlinearity of the response as a function of the irradiation dose. In traditional photo resists, this nonlinearity stems from the solubility behavior of the polymer. Although the catalytic photo process described in this application does not involve a development step, nonlinear behavior was observed. This probably results from a titration effect: a quantity of acid must accumulate before the DMT group is removed.
[0104] The lithographic behavior of the process was evaluated by spin coating a 0.5 μm thick film of poly (methyl methacrylate) (PMMA) containing the nitrobenzyl ester PAC (0.5 wt %) and the enhancer (8 wt %) having the following structures:
onto a glass substrate bearing covalently bound polynucleotides whose terminal 5′ hydroxyl groups were DMT protected. The coated substrate was prebaked at 85° C. for 2 min, irradiated with varying doses at 365 nm, and postba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com